Our promise to you:
Guaranteed product quality, expert customer support.
 24x7 CUSTOMER SERVICE
24x7 CUSTOMER SERVICE
 CONTACT US TO ORDER
CONTACT US TO ORDER
Cat.No. : CSC-RT1202
Host Cell: HEK293T Target Gene: APOE
Size: 1x10^6 cells/vial, 1mL Validation: Sequencing
| Cat. No. | CSC-RT1202 |
| Description | A stable cell line with a homozygous knockout of human APOE using CRISPR/Cas9. |
| Target Gene | APOE |
| Host Cell | HEK293T |
| Host Cell Species | Homo sapiens (Human) |
| Shipping | 10^6 cells/tube |
| Storage | Liquid nitrogen |
| Revival | Rapidly thaw cells in a 37°C water bath. Transfer contents into a tube containing pre-warmed media. Centrifuge cells and seed into a 25 cm2 flask containing pre-warmed media. |
| Media Type | Cells were cultured in DMEM supplemented with 10% fetal bovine serum. |
| Growth Properties | Cells are cultured as a monolayer at 37°C in a humidified atmosphere with 5% CO2. Split at 80-90% confluence, approximately 1:3-1:6. |
| Freeze Medium | Complete medium supplemented with 10% (v/v) DMSO |
| Gene Name | APOE |
| Gene ID | 348 |
| Mycoplasma | Negative |
| Format | One frozen vial containing millions of cells |
| Storage | Liquid nitrogen |
| Safety Considerations |
The following safety precautions should be observed. 1. Use pipette aids to prevent ingestion and keep aerosols down to a minimum. 2. No eating, drinking or smoking while handling the stable line. 3. Wash hands after handling the stable line and before leaving the lab. 4. Decontaminate work surface with disinfectant or 70% ethanol before and after working with stable cells. 5. All waste should be considered hazardous. 6. Dispose of all liquid waste after each experiment and treat with bleach. |
| Ship | Dry ice |
Viruses exploit host cellular machinery to support their replication. Identifying cellular proteins and processes required by viruses during infection is critical for understanding mechanisms of virus-induced disease and for designing host-directed therapies. Here, researchers performed a CRISPR-Cas9-based genome-wide screen in lung epithelial cells infected with PR/8/NS1-GFP virus and used GFPhi cells as a unique screening marker to identify host factors that inhibit influenza A virus (IAV) infection. They found that APOE affects influenza virus infection both in vitro and in vivo. Cellular deficiency of APOE resulted in a significant increase in susceptibility to IAV. Mice lacking APOE exhibited more severe lung pathology, increased viral load, and decreased survival. Mechanistically, the lack of cellular-produced APOE results in impaired cellular cholesterol homeostasis, which enhances influenza virus attachment.
Here, to address the question of where in the viral life cycle the APOE blockade occurs, the researchers first used IAV pseudovirions to determine whether APOE affects IAV entry into cells. APOE knockout cells exhibited increased pseudovirion entry (Figure 1 A and B). Because pseudovirion entry into cells depends on the composition of their envelope, these data support the conclusion that APOE inhibits IAV entry into cells. v-RNA was higher in APOE knockout cells than in WT cells, suggesting that APOE deficiency enhances viral attachment (Figure 1 C). Furthermore, sialidase treatment reduced viral attachment in both WT and APOE knockout cells to levels similar to those detected by RT-PCR (Figure 1D). Together, these findings provide strong evidence that APOE inhibits IAV infection primarily by reducing IAV attachment to its receptors.
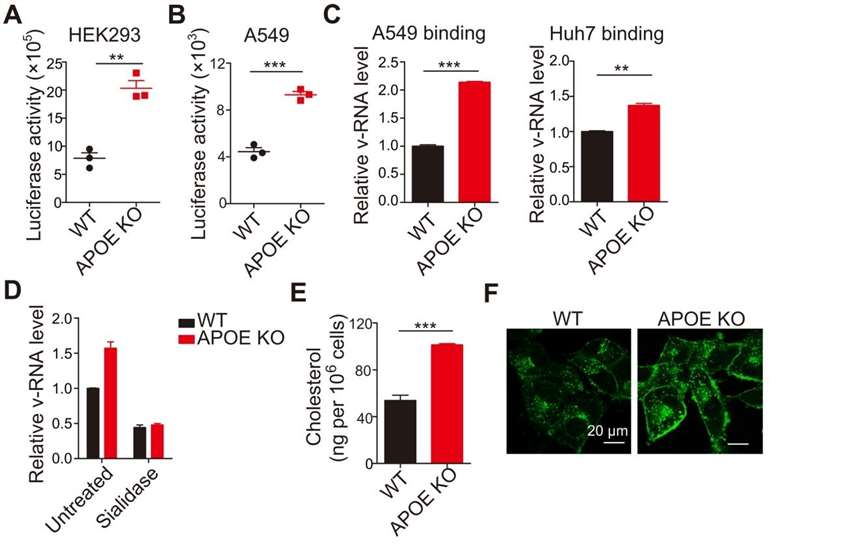 Figure 1. APOE deficiency affects viral attachment. (A and B) HEK293 (A) and A549 (B) WT and APOE KO cells were infected with pseudoviruses. Viral entry was detected by luciferase activity. (C) Viral attachment in WT and APOE KO A549 or Huh7 cells was determined by qRT-PCR. (D) WT and APOE KO A549 cells were pretreated with sialidase and infected as indicated in (C). Viral attachment was determined by qRT-PCR. (E) Total cellular cholesterol in WT or APOE KO A549 cells was detected by Amplex Red Cholesterol assay. (F) Filipin staining in A549 WT or APOE KO cells was detected by confocal microscopy, and representative images are shown. (Gao, Ping, et al. 2022)
Figure 1. APOE deficiency affects viral attachment. (A and B) HEK293 (A) and A549 (B) WT and APOE KO cells were infected with pseudoviruses. Viral entry was detected by luciferase activity. (C) Viral attachment in WT and APOE KO A549 or Huh7 cells was determined by qRT-PCR. (D) WT and APOE KO A549 cells were pretreated with sialidase and infected as indicated in (C). Viral attachment was determined by qRT-PCR. (E) Total cellular cholesterol in WT or APOE KO A549 cells was detected by Amplex Red Cholesterol assay. (F) Filipin staining in A549 WT or APOE KO cells was detected by confocal microscopy, and representative images are shown. (Gao, Ping, et al. 2022)
A: DMEM supplemented with 10% fetal bovine serum.
It is not required to add the selection antibiotics when culturing the KO cells.
A: The knockout cell product is validated by PCR amplification and Sanger Sequencing to confirm the mutation at the genomic level. Please find the detailed mutation info in the datasheet.
A: Single clonal cell.
A: No. This knockout cell product is generated using the CRISPR/Cas9 system to induce small insertions or deletions (indels) resulting in frameshift mutations. Although these frameshift mutations typically disrupt the coding gene, there is a possibility that the non-functional transcript may still be transcribed. Consequently, this could potentially yield misleading results when analyzed by RT-qPCR.
A: The cell line should be stored in liquid nitrogen for long-term preservation.
A: For most cases, we often keep at least 2 clones with different frameshift mutations. Please feel free to contact us to check if there are additional available clones.
If your question is not addressed through these resources, you can fill out the online form below and we will answer your question as soon as possible.
Great! We can use APOE knockout cells to study the role of APOE in Alzheimer's disease and other neurological disorders.
The APOE knockout cell is a cell that has had its APOE gene intentionally removed or knocked out, resulting in a cell that does not produce the APOE protein. I recommend Creative Biogene'cell line.
Write a review of your use of Biogene products and services in your research. Your review can help your fellow researchers make informed purchasing decisions.
Our promise to you:
Guaranteed product quality, expert customer support.
 24x7 CUSTOMER SERVICE
24x7 CUSTOMER SERVICE
 CONTACT US TO ORDER
CONTACT US TO ORDER