Our promise to you:
Guaranteed product quality, expert customer support.
 24x7 CUSTOMER SERVICE
24x7 CUSTOMER SERVICE
 CONTACT US TO ORDER
CONTACT US TO ORDER
Cat.No. : CSC-RT2755
Host Cell: MC38 Target Gene: B2m
Size: 1x10^6 cells/vial, 1mL Validation: Sequencing
| Cat. No. | CSC-RT2755 |
| Description | This cell is a stable cell line with a homozygous knockout of mouse B2m using CRISPR/Cas9. |
| Target Gene | B2m |
| Host Cell | MC38 |
| Host Cell Species | Mus musculus (Mouse) |
| Size Form | 1 vial (>10^6 cell/vial) |
| Shipping | Dry ice package |
| Storage | Liquid Nitrogen |
| Revival | Rapidly thaw cells in a 37°C water bath. Transfer contents into a tube containing pre-warmed media. Centrifuge cells and seed into a 25 cm2 flask containing pre-warmed media. |
| Mycoplasma | Negative |
| Format | One frozen vial containing millions of cells |
| Storage | Liquid nitrogen |
| Safety Considerations |
The following safety precautions should be observed. 1. Use pipette aids to prevent ingestion and keep aerosols down to a minimum. 2. No eating, drinking or smoking while handling the stable line. 3. Wash hands after handling the stable line and before leaving the lab. 4. Decontaminate work surface with disinfectant or 70% ethanol before and after working with stable cells. 5. All waste should be considered hazardous. 6. Dispose of all liquid waste after each experiment and treat with bleach. |
| Ship | Dry ice |
Defective MHC class I antigen presentation is considered the most common mechanism of cancer immune escape. Despite its increasing prevalence, its mechanistic implications and potential strategies to address this challenge remain poorly understood. By studying a mouse tumor model deficient in β2-microglobulin (B2M), researchers found that MHC class I loss leads to immune desertification of the tumor microenvironment (TME) and broad therapeutic resistance to immunotherapy, chemotherapy, and radiotherapy. The study demonstrated that treatment with long-acting mRNA-encoded interleukin 2 (IL2) restored immune cell-infiltrating, IFNγ-promoting, highly pro-inflammatory TME features and, when combined with a tumor-targeting monoclonal antibody (mAb), could overcome therapeutic resistance. Surprisingly, the effectiveness of this treatment was driven by neoantigen-specific IFNγ-releasing CD8+ T cells that recognized neoantigens cross-presented by TME-resident activated macrophages that acquired enhanced antigen presentation capacity and other M1 phenotype-associated features under IL2 treatment. These findings highlight the unexpected importance of restoring neoantigen-specific immune responses in treating MHC class I-deficient cancers.
B2M is a common component of all MHC class I molecules. Its loss results in a complete loss of MHC class I surface expression, resulting in a loss of CD8+ T cell recognition. To investigate MHC class I presentation defects in different settings, the researchers used three mouse B2m knockout tumor cells: CT26, MC38, and B16F10. Among them, CT26 and MC38 form highly immunogenic tumors and trigger spontaneous CD8+ T cell responses, while B16F10 melanoma has a low prevalence of tumor-infiltrating leukocytes (TILs) and is considered a non-immunogenic tumor. B2m knockout (B2m-/-) cells lack MHC class I surface expression and therefore cannot be recognized by co-cultured antigen-specific CD8+ T cells (Figure 1A and B). In syngeneic mice, longitudinal analysis of immune cell infiltration during the growth of wild-type and B2m-/- tumors revealed a gradual reduction in immune cell infiltration in CT26-B2m-/- tumors, with immune cell desertification of the tumor microenvironment (TME) within 20 days after inoculation, and a reduction in CD8+ T cells, NK cells, and conventional type I dendritic cells (cDC1) (Figure 1C). B2M knockout had similar effects on MC38 tumors, while B16F10 tumors, regardless of their B2m genotype, showed sparse immune infiltration (Figures 1C and D). CT26-B2m knockout and MC38-B2m knockout tumors showed significantly faster progression in vivo, but not in in vitro cell culture conditions (Figures 1F and G), while B16F10 tumors showed no significant growth differences compared with wild-type tumors (Figure 1F).
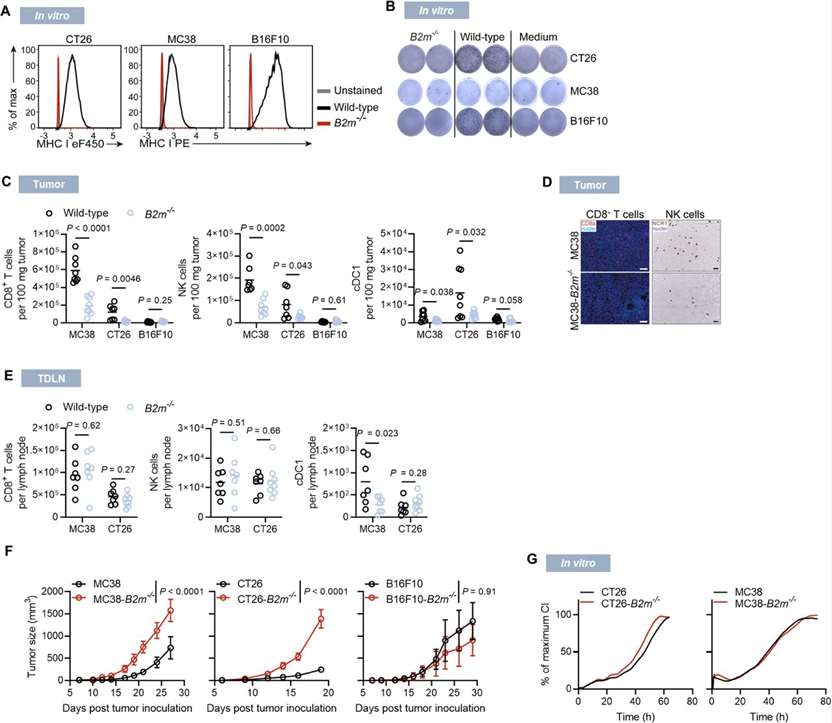 Figure 1. Characterization of MHC class I-deficient tumors. (Beck J D, et al., 2023)
Figure 1. Characterization of MHC class I-deficient tumors. (Beck J D, et al., 2023)
A: The knockout cell product is validated by PCR amplification and Sanger Sequencing to confirm the mutation at the genomic level. Please find the detailed mutation info in the datasheet.
A: Single clonal cell.
A: No. This knockout cell product is generated using the CRISPR/Cas9 system to induce small insertions or deletions (indels) resulting in frameshift mutations. Although these frameshift mutations typically disrupt the coding gene, there is a possibility that the non-functional transcript may still be transcribed. Consequently, this could potentially yield misleading results when analyzed by RT-qPCR.
A: The cell line should be stored in liquid nitrogen for long-term preservation.
A: For most cases, we often keep at least 2 clones with different frameshift mutations. Please feel free to contact us to check if there are additional available clones.
If your question is not addressed through these resources, you can fill out the online form below and we will answer your question as soon as possible.
The MC38 Mouse B2m Knockout cell line provides an advanced model for studying immuno-oncology. Its unique genetic knockout allows for detailed exploration of tumor-immune interactions, making it an indispensable tool in our quest to develop novel therapeutic strategies.
The cells are robust and adapt well to a variety of experimental conditions, reducing the need for extensive optimization steps and saving precious research time.
Write a review of your use of Biogene products and services in your research. Your review can help your fellow researchers make informed purchasing decisions.
Our promise to you:
Guaranteed product quality, expert customer support.
 24x7 CUSTOMER SERVICE
24x7 CUSTOMER SERVICE
 CONTACT US TO ORDER
CONTACT US TO ORDER