Transfected Stable Cell Lines
Reliable | High-Performance | Wide Rage
Precision reporter, kinase, immune receptor, biosimilar, Cas9, and knockout stable cell lines for diverse applications.
Transfected Stable Cell Lines
Reliable | High-Performance | Wide Rage
Precision reporter, kinase, immune receptor, biosimilar, Cas9, and knockout stable cell lines for diverse applications.
Premade Virus Particles
Ready-to-Use | High Titer | Versatile Applications
Premade AAV, adenovirus, lentivirus particles, safe, stable, in stock.
Virus-Like Particles (VLPs)
Stable | Scalable | Customizable
Advanced VLPs for vaccine development (Chikungunya, Dengue, SARS-CoV-2), gene therapy (AAV1 & AAV9), and drug screening (SSTR2, CCR5).
Oligonucleotide Products
Precise | High Yield | Tailored Solutions
Accelerate your research with cost-effective LncRNA qPCR Array Technology.
RNA Interference Products
Targeted | Potent | High Specificity
Human Druggable Genome siRNA Library enables efficient drug target screening.
Recombinant Drug Target Proteins
Authentic | Versatile | Accelerated
Providing functional, high-purity recombinant proteins—including membrane proteins and nanodiscs—to overcome bottlenecks in drug screening and target validation.
Clones
Validated | Reliable | Comprehensive Collection
Ready-to-use clones for streamlined research and development.
Kits
Complete | Convenient | High Sensitivity
Chromogenic LAL Endotoxin Assay Kit ensures precise, FDA-compliant endotoxin quantification for biosafety testing.
Enzymes
Purified | Stable | Efficient
Powerful Tn5 Transposase for DNA insertion and random library construction.
Aptamers
Highly Specific | Robust | Versatile
Aptamers for key proteins like ACVR1A, Akt, EGFR, and VEGFR.
Adjuvants
Enhancing | Synergistic | Effective
Enhance immune responses with high-purity, potent CpG ODNs.
Laboratory Equipment
Innovative | Reliable | High-Precision
Effortlessly streamline DNA extraction with CB™ Magnetic-Nanoparticle Systems.
Stable Cell Line Generation
Reliable | Scalable | Customizable
Fast proposals, regular updates, and detailed reports; strict quality control, and contamination-free cells; knockout results in 4-6 weeks.
Target-based Drug Discovery Service
Innovative | Comprehensive | Efficient
Target identification, validation, and screening for drug discovery and therapeutic development.
Custom Viral Service
Versatile | High-Yield | Safe
Unbeatable pricing, fully customizable viral packaging services (covering 30,000+ human genes, 200+ mammals, 50+ protein tags).
Custom Antibody Service
Precise | Flexible | Efficient
End-to-end antibody development support, from target to validation, enabling clients to rapidly obtain application-ready antibodies.
Antibody-Drug Conjugation Service
Integrated | Controlled | Translational
Comprehensive solutions covering design, development, and validation to ensure conjugated drugs with consistent quality and clinical potential.
Protein Degrader Service
Efficient | High-Precision | Advanced Therapeutics
Harness the power of protein degraders for precise protein degradation, expanding druggable targets and enhancing therapeutic effectiveness for cutting-edge drug discovery.
Nucleotides Service
Accurate | Flexible | High-Quality
Custom synthesis of oligonucleotides, primers, and probes for gene editing, PCR, and RNA studies.
Custom RNA Service
Custom RNA ServicePrecise | Flexible | GMP-ReadyCustom
RNA design, synthesis, and manufacturing—covering mRNA, saRNA, circRNA, and RNAi. Fast turnaround, rigorous QC, and seamless transition from research to GMP production.
Custom Libraries Construction Service
Comprehensive | High-throughput | Accurate
Custom cDNA, genomic, and mutagenesis libraries for drug discovery, screening, and functional genomics.
Gene Editing Services
Precise | Efficient | Targeted
Gene editing solutions for gene editing, knockouts, knock-ins, and customized genetic modifications. Integrated multi-platform solutions for one-stop CRISPR sgRNA library synthesis and gene screening services
Microbe Genome Editing Service
Precise | Scalable | Customizable
Enhance microbial productivity with advanced genome editing using Rec-mediated recombination and CRISPR/Cas9 technologies.
Biosafety Testing Service
Reliable | Comprehensive | Regulated
Complete biosafety testing solutions for gene therapy, viral vectors, and biologics development.
Plant Genetic Modification Service
Advanced | Sustainable | Tailored
Genetic modification for crop improvement, biotechnology, and plant-based research solutions.
Plant-based Protein Production Service
Efficient | Scalable | Customizable
Plant-based protein expression systems for biopharmaceuticals, enzyme production, and research.
Aptamers Service
Innovative | Fast | Cost-Effective
Revolutionizing drug delivery and diagnostic development with next-generation high-throughput aptamer selection and synthesis technologies.
CGT Biosafety Testing
Comprehensive | Accurate | Regulatory-compliant
Internationally certified evaluation system for biologics, gene therapies, nucleic acid drugs, and vaccines.
Pandemic Detection Solutions
Rapid | Precise | Scalable
Balancing accuracy, accessibility, affordability, and rapid detection to safeguard public health and strengthen global response to infectious diseases.
cGMP Cell Line Development
Reliable | Scalable | Industry-leading
Stable expression over 15 generations with rapid cell line development in just 3 months.
Supports adherent and suspension cell lines, offering MCB, WCB, and PCB establishment.
GMP mRNA Production
Efficient | Scalable | Precise
Scalable mRNA production from milligrams to grams, with personalized process design for sequence optimization, cap selection, and nucleotide modifications, all in one service.
GMP Plasmid Production
High Quality | Scalable | Regulatory-compliant
Our plasmid production services span Non-GMP, GMP-Like, and GMP-Grade levels, with specialized options for linearized plasmids.
GMP Viral Vector Manufacturing
Scalable | High Yield | Quality-driven
Advanced platforms for AAV, adenovirus, lentivirus, and retrovirus production, with strict adherence to GMP guidelines and robust quality control.
AI-Driven Gene Editing and Therapy
Innovative | Precision | Transformative
AI-powered one-click design for customized CRISPR gene editing strategy development.
AI-Antibody Engineering Fusion
Next-Generation | Targeted | Efficient
AI and ML algorithms accelerate antibody screening and predict new structures, unlocking unprecedented possibilities in antibody engineering.
AI-Driven Enzyme Engineering
Smart | Efficient | Tailored
High-throughput enzyme activity testing with proprietary datasets and deep learning models for standardized and precise enzyme engineering design.
AI-Enhanced Small Molecule Screening
Predictive | Efficient | Insightful
Leverage AI to uncover hidden high-potential small molecules, prioritize leads intelligently, and reduce costly trial-and-error in early drug discovery.
AI-Driven Protein Degrader Drug Development
Innovative | Targeted | Accelerated
Use AI-guided design to optimize protein degraders, addressing design complexity and enhancing efficacy while shortening development timelines.
| Cat.No. | Product Name | Price |
|---|
| Cat.No. | Product Name | Price |
|---|
| Cat.No. | Product Name | Price |
|---|
| Cat.No. | Product Name | Price |
|---|
KRAS is the most common type of mutation in the RAS family. The Kras mutation will cause it to lose GTP hydrolase activity, thereby continuously activating downstream signaling pathways, causing cell proliferation to be uncontrolled and cancerous. At the same time, Kras mutation is a necessary condition for the growth and proliferation of tumor cells, and it is also one of the key reasons for tumor acquired resistance. KRAS is an important member of the RAS family and has a high mutation rate and has been receiving much attention. At present, most of the KRAS downstream effect pathways are RAF-MEK-ERK, PI3K-AKT and RalGDS-Ral.
KRAS Downstream Signaling Pathway
As one of the mitogen-activated protein kinase (MAPK) signaling pathways, the RAS-RAF-MEKERK signaling pathway is a key pathway in many signaling pathways that control cell growth, proliferation, differentiation and apoptosis. The cycle progression of different types of cells and apoptosis, signal molecule mutations in the pathway are often closely related to human cancer, and inhibitors developed for key molecules in the signaling pathway are also widely used in clinical cancer treatment. This pathway is activated by growth factors, mitogens, antigen receptors or GPCR (guanosine-binding protein coupled receptor), and transmits extracellular signals into the nucleus. When GTP replaces GDP binding to KRAS, KRAS is activated, and GTP-bound KRAS recruits RAF (rapidly accelerated fibrosarcoma) to the plasma membrane and activates RAF protein kinases, including CRAF, BRAF, and ARAF. The activated RAF will phosphorylate the serine/threonine residues of the bispecific kinases MEK1 and MEK2, which in turn leads to activation of MEK. The threonine and tyrosine residues of ERK can be further phosphorylated to activate them. Activated ERK can phosphorylate cytosolic signaling proteins, including p90 ribosomal S6 kinase (RSK) and MAPK-interacting serine/threonine kinase (MNK). It can also be transferred to the nucleus to directly activate cAMP response selment binding protein (CREB), c-Jun, c-Fos and other transcription factors to regulate cell growth, proliferation and differentiation.
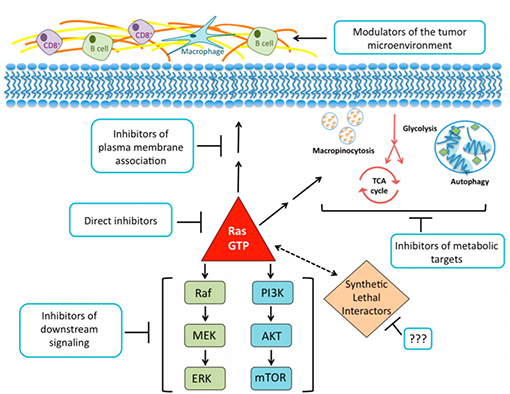 Figure 1. The current paths in the pursuit of an anti‐KRAS therapy. (Daniel, Z., et al. 2016)
Figure 1. The current paths in the pursuit of an anti‐KRAS therapy. (Daniel, Z., et al. 2016)
Phosphatidylinositol-4, 5-bisphosphate 3-kinase (PI3K) is also a downstream effector of KRAS and can be activated by RAS to participate in the regulation of cell proliferation, differentiation, apoptosis and glucose transport.
Kras Mutation and Tumor Development
Activation mutations in the Kras gene are closely related to the development of human malignancies and tumor recurrence. Genetic and biochemical studies have demonstrated that KRAS-dependent signaling plays an important role in regulating the growth, proliferation, invasion and metastasis of a variety of cancer cells. The study found that in non-small cell lung cancer, the prevalence of Kras mutations in Caucasian patients is 20% to 30%. At the same time, studies have shown that Kras mutations are related to gender, age, etc. Kras mutations occur more frequently in women and younger patients. In a study of patients with colorectal cancer, Kras mutations were also more likely to occur in women and younger patients. At the same time, pathological studies have shown that Kras mutations are more common in patients with lung adenocarcinoma, especially invasive mucinous adenocarcinoma.
The smoking status is also related to the presence of the Kras mutation and the type of mutation. Studies have shown that using the allele-specific ligation method to detect Kras mutations in primary tumors, 92 (87%) of 106 patients with lung adenocarcinoma are smokers, so compared to non-smokers, Kras Mutations are more common in smoking patients. KRAS is also considered a marker in patient prognosis. In patients with non-small cell lung cancer, patients with Kras mutations have shorter survival than patients with Kras wild type, especially those with G12C point mutations. Other studies have shown that in colorectal cancer, mutations in the Kras codon 13 have an adverse effect on patient survival.
Kras Mutant Tumor Treatment Strategy
Studies have shown that targeting wild-type Kras by RNA interference (RNAi) can significantly inhibit tumor cell growth in lung cancer and colorectal cancer. Studies have also shown that exosomes secreted by normal fibroblast-like mesenchymal cells are engineered and packaged for delivery of siRNA or shRNA against the KrasG12D mutant. Compared to liposomes, engineered exosomes (iExosomes) is able to effectively target KrasG12D in vivo, providing a reliable method for direct targeted treatment of Kras mutant tumors.
In the strategy of treating Kras mutant tumors, co-inhibition of RAF-MEK-ERK and PI3KAKT-mTOR signaling pathways often achieve better clinical outcomes. In a clinical phase I trial, the PI3K inhibitor GDC-0941 was combined with the MEK inhibitor GDC-0973 to treat patients with non-small cell lung cancer with a Kras mutation and responded well.
References:
Contact us today for a free consultation with the scientific team and discover how Creative Biogene can be a valuable resource and partner for your organization.
Inquiry

Copyright © Creative Biogene. All rights reserved.