Transfected Stable Cell Lines
Reliable | High-Performance | Wide Rage
Precision reporter, kinase, immune receptor, biosimilar, Cas9, and knockout stable cell lines for diverse applications.
| Cat.No. | Product Name | Price |
|---|---|---|
| CSC-DC001523 | Panoply™ Human BRCA2 Knockdown Stable Cell Line | Inquiry |
| CSC-RK0027 | Human BRCA2 Knockdown Cell Line-HeLa | Inquiry |
| CSC-RT0015 | Human BRCA2 Knockout Cell Line-DLD-1 | Inquiry |
| CSC-RT0028 | Human BRCA2 Knockout Cell Line-DLD-1 | Inquiry |
| CSC-RT2263 | Human BRCA2 Knockout Cell Line-HEK293T | Inquiry |
| CSC-RT2625 | Human BRCA2 Knockout Cell Line-LNCaP | Inquiry |
| CSC-RT2626 | Human BRCA2 Knockout Cell Line-PC3M | Inquiry |
| CSC-SC001523 | Panoply™ Human BRCA2 Over-expressing Stable Cell Line | Inquiry |
| Cat.No. | Product Name | Price |
|---|---|---|
| CSC-DC001523 | Panoply™ Human BRCA2 Knockdown Stable Cell Line | Inquiry |
| CSC-RK0027 | Human BRCA2 Knockdown Cell Line-HeLa | Inquiry |
| CSC-RT0015 | Human BRCA2 Knockout Cell Line-DLD-1 | Inquiry |
| CSC-RT0028 | Human BRCA2 Knockout Cell Line-DLD-1 | Inquiry |
| CSC-RT2263 | Human BRCA2 Knockout Cell Line-HEK293T | Inquiry |
| CSC-RT2625 | Human BRCA2 Knockout Cell Line-LNCaP | Inquiry |
| CSC-RT2626 | Human BRCA2 Knockout Cell Line-PC3M | Inquiry |
| CSC-SC001523 | Panoply™ Human BRCA2 Over-expressing Stable Cell Line | Inquiry |
| Cat.No. | Product Name | Price |
|---|---|---|
| CSC-DC001523 | Panoply™ Human BRCA2 Knockdown Stable Cell Line | Inquiry |
| CSC-RK0027 | Human BRCA2 Knockdown Cell Line-HeLa | Inquiry |
| CSC-RT0015 | Human BRCA2 Knockout Cell Line-DLD-1 | Inquiry |
| CSC-RT0028 | Human BRCA2 Knockout Cell Line-DLD-1 | Inquiry |
| CSC-RT2263 | Human BRCA2 Knockout Cell Line-HEK293T | Inquiry |
| CSC-RT2625 | Human BRCA2 Knockout Cell Line-LNCaP | Inquiry |
| CSC-RT2626 | Human BRCA2 Knockout Cell Line-PC3M | Inquiry |
| CSC-SC001523 | Panoply™ Human BRCA2 Over-expressing Stable Cell Line | Inquiry |
| Cat.No. | Product Name | Price |
|---|---|---|
| CSC-DC001523 | Panoply™ Human BRCA2 Knockdown Stable Cell Line | Inquiry |
| CSC-RK0027 | Human BRCA2 Knockdown Cell Line-HeLa | Inquiry |
| CSC-RT0015 | Human BRCA2 Knockout Cell Line-DLD-1 | Inquiry |
| CSC-RT0028 | Human BRCA2 Knockout Cell Line-DLD-1 | Inquiry |
| CSC-RT2263 | Human BRCA2 Knockout Cell Line-HEK293T | Inquiry |
| CSC-RT2625 | Human BRCA2 Knockout Cell Line-LNCaP | Inquiry |
| CSC-RT2626 | Human BRCA2 Knockout Cell Line-PC3M | Inquiry |
| CSC-SC001523 | Panoply™ Human BRCA2 Over-expressing Stable Cell Line | Inquiry |
BRCA2 is a human tumor suppressor gene, found in all humans; its protein, also called by the synonym breast cancer type 2 susceptibility protein, is responsible for repairing DNA.
Genomic instability is at the heart of aging, cancer and other diseases. Not only proteins involved in DNA replication or the DNA damage response that are important for maintaining the integrity of the genome. From yeastto higher eukaryotes, mutations in pre-mRNA splicing and in the biogenesis and export of messenger ribonucleo protein (mRNP) also induce DNA damage and genome instability. This instability is frequently arised from R-loops. R-loop is a three-stranded nucleic acid structure, composed of a DNA: RNA hybrid and the associated non-template single-stranded DNA, is formed by DNA-RNA hybrids and a displaced single-stranded DNA. The human TREX-2 complex, is involved in mRNP biogenesis and export, prevents genome instability. In cells depleted of the TREX-2 subunits PCID2, GANP and DSS1, γ-H2AX (Ser-139 phosphorylated histone H2AX) and 53BP1 are determined accumulated. The BRCA2 repair factor, which binds to DSS1, also associates with PCID2 in the cell. In TREX-2-depleted cells can’t detect R-loops, but did detect the accumulation of R-loops in BRCA2-depleted cells, indicating that loops are frequently formed in cells and that BRCA2 is required for their processing. This link between BRCA2 and RNA-mediated genome instability suggests that R-loops may be the main source of replication stress and cancer-associated instability.
Bhatia, V., et al. proposed a model to explain the role of BRCA2 preventing R-loops as a source of genome instability. In the absence of TREX-2, other RNA-binding factors help to prevent R-loop accumulation to a level detectable in the conditions tested. Most likely that TREX-2 helps recruit BRCA2 to the proximity of transcribed regions, where BRCA2 binds to the branched structure formed by the displaced ssDNA and the DNA-RNA hybrids of naturally formed R-loops (Fig. 1a). Such a structure may mimic the replicative intermediate to which BRCA2 is believed to bind in vivo. In replicating chromatin, because BRCA2 and BRCA1 work in the Fanconi anaemia pathway, which removes crosslinks and replication-blocking lesions, RFs encountering an R-loop could target the action of BRCA2, BRCA1 and possibly other Fanconi anaemia proteins to prevent fork collapse and reversal, probably impeding R-loop extension, and could promote restarting of the replication fork and dissolution of the R-loop (Fig. 1b). Thus, TREX-2 couldfunction at co-transcriptional R-loopsncountering RFs by regulating the stability and/or recruitment of BRCA2 to the ssDNA substrates generated at the sites of collisions.
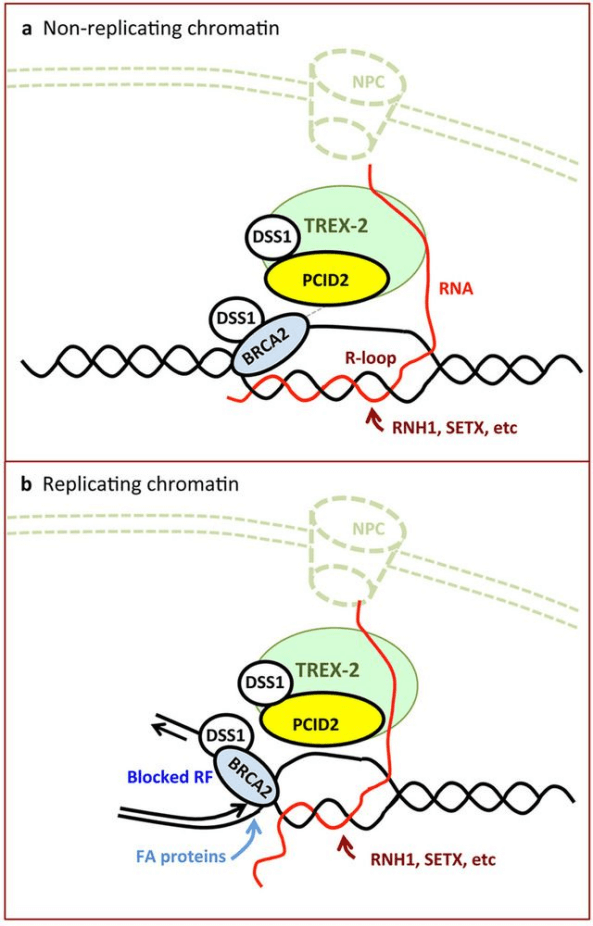 Fig. 1. Model to explain the role of BRCA2 preventing R-loops as a source of genome instability. (Bhatia, V., et al. (2014).)
Fig. 1. Model to explain the role of BRCA2 preventing R-loops as a source of genome instability. (Bhatia, V., et al. (2014).)
Furthemore BRCA2 exerts its tumor suppressive function in the cell nucleus through its role in the repair of DNA breaks by homologous recombination. ATM is a serine/threonine protein kinase that is recruited and activated by DNA double-strand breaks. It phosphorylates several key proteins that initiate activation of the DNA damage repair or apoptosis. DNA double-strand damages also induce the activation of Fanconi anemia core complex. The FA proteins FANC-A, B, C, E, F, G and L (Fanconi anaemia complementation group) assemble in a nuclear complex required for activation through monoubiquitination of FANCD2 and FANCI in response to DNA damage. FANCL provides the monoubiquitin E3 ligase function for both FANCI and FANCD2. FANCD2’s activite indicate the activite of FA pathway. After DNA damage, ATM and ATR are required for FANCD2 phosphorylation. ATM also phosphorylated CHEK2, the activated Chk2 kinase can act as a signal transducer and phosphorylate a variety of substrates, including the Cdc25 phosphatases, p53, PML, E2F-1, and BRCA1. Ubiquinated FANCD2 complexes with BRCA1 and RAD51 in S-phase-specific nuclear foci. Toshiyasu, et al. suggested that FANCD2/BRCA1 complexes and FANCD2/RAD51 complexes participate in an S-phase-specific cellular process, such as DNA repair by homologous recombination.
The PALB2 protein acts as a hub, bringing together BRCA1, BRCA2 and RAD51 at the site where the DNA double strand breaks, and also binds to RAD51C, a member of the RAD51 paralog complex RAD51B-RAD51C-RAD51D-XRCC2 (BCDX2). The BCDX2 complex is responsible for RAD51 recruitment or stabilization at damage sites. RAD51 plays a key role in DNA homologous recombinational repair during double strand break. BRCA2 controls the accumulation of the RAD51 recombinase enzyme at sites of DNA breakage in the nucleus, nucleates RAD51 filament formation at single-strand-double-strand DNA junctions, and promotes RAD51 binding on ssDNA whilst inhibiting dsDNA binding. A nuclear export signal (NES) in BRCA2 is masked by its interaction with the small (70 amino acids (aa)), acidic protein DSS1. BRCA2D2723H point mutations impairing BRCA2-DSS1 binding render BRCA2 cytoplasmic. In turn, cytoplasmic mis-localization of mutant BRCA2 inhibits the nuclear retention of RAD51, by exposing a similar NES in RAD51 usually obscured by the BRCA2-RAD51 interaction.
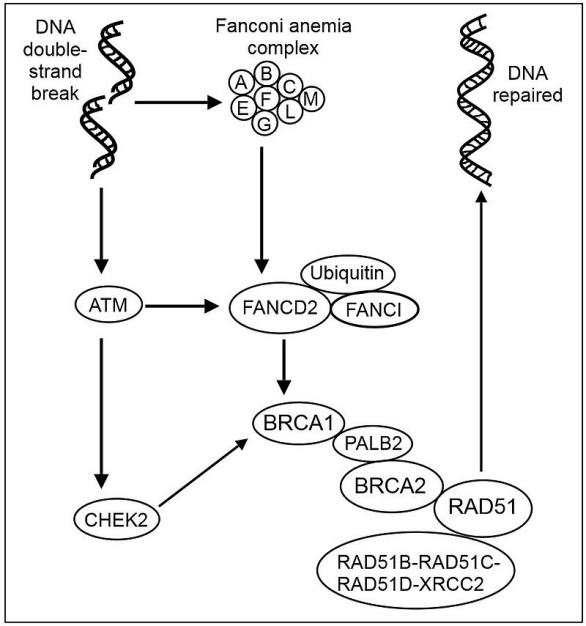 Fig 2. Recombinational repair of DNA double-strand damage - some key steps. (By Chaya5260 [CC BY-SA 4.0 (https://creativecommons.org/licenses/by-sa/4.0)], from Wikimedia Commons).
Fig 2. Recombinational repair of DNA double-strand damage - some key steps. (By Chaya5260 [CC BY-SA 4.0 (https://creativecommons.org/licenses/by-sa/4.0)], from Wikimedia Commons).
References:
If you don’t find the cell line you want, Creative Biogene can also provide stable cell line generation service with the best prices and fastest turnaround time for you! Contact us for more information or to request a quote.
Inquiry