Transfected Stable Cell Lines
Reliable | High-Performance | Wide Rage
Precision reporter, kinase, immune receptor, biosimilar, Cas9, and knockout stable cell lines for diverse applications.
| Cat.No. | Product Name | Price |
|---|---|---|
| CSC-DC001522 | Panoply™ Human BRCA1 Knockdown Stable Cell Line | Inquiry |
| CSC-RK0030 | Human BRCA1 Knockdown Cell Line-HeLa | Inquiry |
| CSC-RK0252 | Human BRCA1 Knockdown Cell Line - Glioma | Inquiry |
| CSC-RO02547 | Human BRCA1 Stable Cell Line - MDA-MB-436 | Inquiry |
| CSC-SC001522 | Panoply™ Human BRCA1 Over-expressing Stable Cell Line | Inquiry |
| Cat.No. | Product Name | Price |
|---|---|---|
| CSC-DC001522 | Panoply™ Human BRCA1 Knockdown Stable Cell Line | Inquiry |
| CSC-RK0030 | Human BRCA1 Knockdown Cell Line-HeLa | Inquiry |
| CSC-RK0252 | Human BRCA1 Knockdown Cell Line - Glioma | Inquiry |
| CSC-RO02547 | Human BRCA1 Stable Cell Line - MDA-MB-436 | Inquiry |
| CSC-SC001522 | Panoply™ Human BRCA1 Over-expressing Stable Cell Line | Inquiry |
| Cat.No. | Product Name | Price |
|---|---|---|
| CSC-DC001522 | Panoply™ Human BRCA1 Knockdown Stable Cell Line | Inquiry |
| CSC-RK0030 | Human BRCA1 Knockdown Cell Line-HeLa | Inquiry |
| CSC-RK0252 | Human BRCA1 Knockdown Cell Line - Glioma | Inquiry |
| CSC-RO02547 | Human BRCA1 Stable Cell Line - MDA-MB-436 | Inquiry |
| CSC-SC001522 | Panoply™ Human BRCA1 Over-expressing Stable Cell Line | Inquiry |
| Cat.No. | Product Name | Price |
|---|---|---|
| CSC-DC001522 | Panoply™ Human BRCA1 Knockdown Stable Cell Line | Inquiry |
| CSC-RK0030 | Human BRCA1 Knockdown Cell Line-HeLa | Inquiry |
| CSC-RK0252 | Human BRCA1 Knockdown Cell Line - Glioma | Inquiry |
| CSC-RO02547 | Human BRCA1 Stable Cell Line - MDA-MB-436 | Inquiry |
| CSC-SC001522 | Panoply™ Human BRCA1 Over-expressing Stable Cell Line | Inquiry |
BRCA1 was identified in early 1990’s as tumor suppressor, which is known to play a huge role in sustaining the genome stability in many different tissues. BRCA1 mutations are inheritable and the mutation associated with many cancers, such as breast cancer and ovarian cancer. There's a 50–60% chance of getting breast cancer and 50% risk of developing ovarian cancer for BRCA1mutation carriers. BRCA1 mutant tumors showed a high prevalence in the age group of 30 to 50 years. Although the cause of the early onset is unclear, it is generally believed that hormonal factors play a huge role in inducing BRCA1 related breast tumors. The cause for the tissue specific tumorigenecity in BRCA1 mutation could be linked to estrogen receptorα (ER-α) which is repressed by BRCA1 and mutation in BRCA1 leads to ER-α mediated tumorigenesis as breast/ovarian tissues are under the control of exposure to estrogen that is expressed during menstrual cycle.
BRCA1 combines with other tumor suppressors
Yasmeen et al. has shown that the presence of mutant type BRCA1 down regulated the expression of E-cadherin. The reduction of e-cadherin expression has been a hallmark of the EMT process and is associated with the migration and invasion of breast cancer cells. The key transcription factors Snail 1, Snail 2, ZEB 1, Ets-1, FOXC2 and Twist are acquired during the process of EMT. Snail is a downstream target of many signaling pathways that leads to EMT during cancer and in fact EMT is considered as a rare event without the activation of Snail/Slug. In breast cancer, the expression of slug is up regulated in highly aggressive basal breast tumors in comparison to luminal breast tumors. Also, the over expression of slug is reported to induce basal phenotype in breast cancer harboring luminal phenotype and vice versa which ultimately drives the process of EMT. In breast tumors which are BRCA1 mutated, slug expression is up regulated and in breast tissues which harbor wild type BRCA1 slug expression is down regulated.
Cytoskeletal proteins play a dominant role during EMT in all types of breast cancers. The over expression and under expression of certain cytoskeletal proteins in BRCA1 mutated tumors confers the basal like aggressive behavior of the tumorThe key cytoskeletal proteins that are linked with the process of EMT are β-catenin, vimentin and cytokeratins, where former two molecules are induced and cytokeratins are attenuated during EMT.
Micro RNA’s are 18-25 nucleotides small non coding RNA’s which play a myriad of functions in the normal and cancerous tissues by post transcriptional regulation of critical genes. There are many evidences show that micro RNA can relate breast cancer with BRCA1. Many micro RNA’s such as miR-205, miR-155, miR-99b can be regulated by BRCA1 and also a few micro RNA’s regulating the expression of BRCA1 such as miR-146a, 146b, miR-182, miR-638, miR-17, miR-335, miR-342 is also reported.
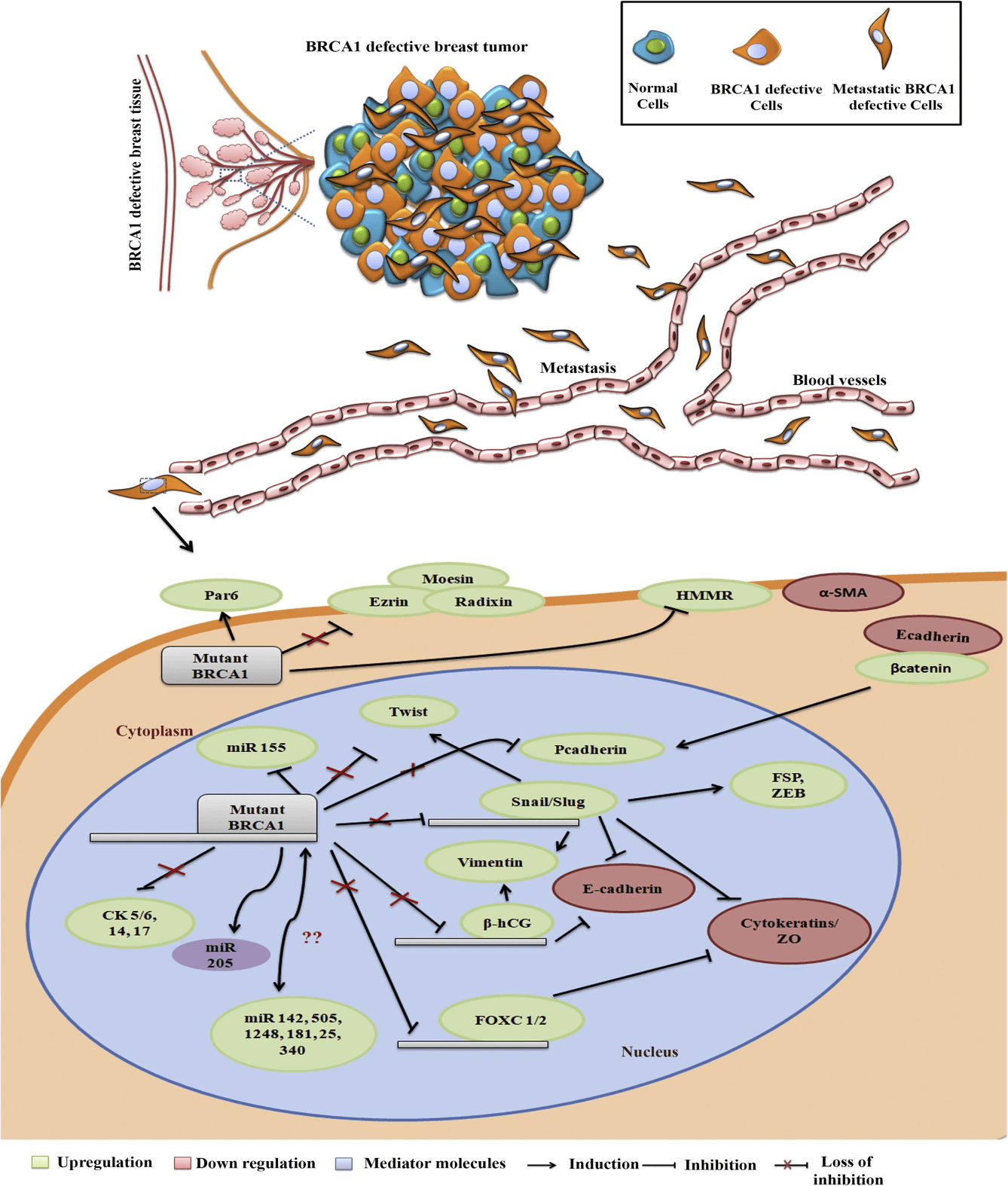 Fig 1. Regulation of EMT molecules in BRCA1 wild type and BRCA1 mutant breast cancer cells. (S.K. Sengodan, et al. 2018).
Fig 1. Regulation of EMT molecules in BRCA1 wild type and BRCA1 mutant breast cancer cells. (S.K. Sengodan, et al. 2018).
BRCA1 play a key role in DNA damage repair
External and internal hazards including reactive oxygen species, ionizing radiation (IR) and many chemicals can induce DNA double strand breaks (DSBs). Various cell cycle checkpoints are activated in response to DSBs or paused replication forks. These checkpoints are considered to coordinate cell cycle progression with DNA repair or induce apoptosis when DNA damage cannot be repaired promptly. Therefore, DNA damage checkpoints are considered important self-defense mechanisms to ensure genome stability. Many proteins including the protein kinase ataxiatelangiectasia mutated (ATM), γH2AX, mediator of DNA damage checkpoint protein 1 (MDC1), Nijmegen breakage syndrome 1 (NBS1), the product of Breast Cancer Susceptibility Gene 1 (BRCA1), and checkpoint kinases 1 and 2 (Chk1 and Chk2) are involved in the ionizing radiation (IR)-induced DNA damage response pathway.
The BRCA1 gene encodes all 1863 amino acid protein, which contains two specific regions, the N-terminal Ring domain that has E3 ligase activity and the tandem C terminal BRCT motifs that specifically recognize phosphoserine/ theronine motifs. Following DNA damage, the key upstream sensor ATM initiates the DNA damage response. ATM is activated and autophosphorylated at several residues including Ser 1981 in response to DNA double stranded breaks. As soon as ATM activation, ATM can phosphorylate a histone H2A variant H2AX at residue Ser 139 at or near the sites of DNA breaks. Hence phosphorylated H2AX has been widely used as a marker of DNA double stranded breaks. This phosphorylated H2AX can bind directly to MDC1 BRCT domains and thus accumulate MDC1 to DNA damage sites. MDC1 can directly or indirectly recruit additional active ATM to the sites of DNA damage. This accumulation of active ATMs at DSBs should result in the further phosphorylation of H2AX at mega-base region surrounding any single DNA break and provide docking sites not only for MDC1, but also other checkpoint proteins at or near the sites of DNA breaks. We believe that this positive feedback mechanism amplifies the DNA damage signals and is especially critical for checkpoint activation following low doses of DNA damage.
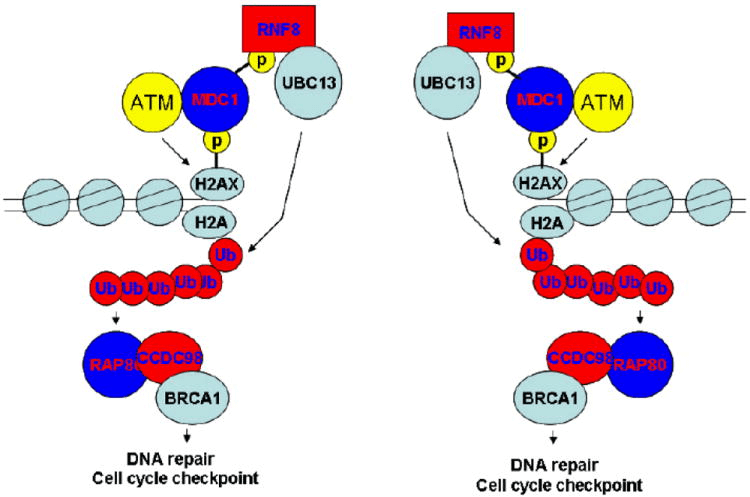 Fig 2. A proposed model of the DNA-damage response pathway (Kim and Chen. 2008).
Fig 2. A proposed model of the DNA-damage response pathway (Kim and Chen. 2008).
References:
If you don’t find the cell line you want, Creative Biogene can also provide stable cell line generation service with the best prices and fastest turnaround time for you! Contact us for more information or to request a quote.
Inquiry