Transfected Stable Cell Lines
Reliable | High-Performance | Wide Rage
Precision reporter, kinase, immune receptor, biosimilar, Cas9, and knockout stable cell lines for diverse applications.
| Cat.No. | Product Name | Price |
|---|---|---|
| CLKO-1589 | BRAF KO Cell Lysate-HeLa | Inquiry |
| CSC-DC001519 | Panoply™ Human BRAF Knockdown Stable Cell Line | Inquiry |
| CSC-RO0415 | Human BRAF-V600E Stable Cell Line - BaF3 | Inquiry |
| CSC-RO0732 | Human BRAF-K601N Stable Cell Line - Ba/F3 | Inquiry |
| CSC-RO0733 | Human BRAF-N486-P490del-K547Q Stable Cell Line - Ba/F3 | Inquiry |
| CSC-RO0734 | Human BRAF-V600_K601delinsE Stable Cell Line - Ba/F3 | Inquiry |
| CSC-RO0735 | Human BRAF-T599_V600insT Stable Cell Line - Ba/F3 | Inquiry |
| CSC-RO0768 | Human BRAF-G469A Stable Cell Line - Ba/F3 | Inquiry |
| CSC-RT1742 | Human BRAF Knockout Cell Line-HeLa | Inquiry |
| CSC-RT2767 | Human BRAF Knockout Cell Line-HCT116 | Inquiry |
| CSC-RT2768 | Human BRAF Knockout Cell Line-DLD1 | Inquiry |
| CSC-SC001519 | Panoply™ Human BRAF Over-expressing Stable Cell Line | Inquiry |
| Cat.No. | Product Name | Price |
|---|---|---|
| CLKO-1589 | BRAF KO Cell Lysate-HeLa | Inquiry |
| CSC-DC001519 | Panoply™ Human BRAF Knockdown Stable Cell Line | Inquiry |
| CSC-RO0415 | Human BRAF-V600E Stable Cell Line - BaF3 | Inquiry |
| CSC-RO0732 | Human BRAF-K601N Stable Cell Line - Ba/F3 | Inquiry |
| CSC-RO0733 | Human BRAF-N486-P490del-K547Q Stable Cell Line - Ba/F3 | Inquiry |
| CSC-RO0734 | Human BRAF-V600_K601delinsE Stable Cell Line - Ba/F3 | Inquiry |
| CSC-RO0735 | Human BRAF-T599_V600insT Stable Cell Line - Ba/F3 | Inquiry |
| CSC-RO0768 | Human BRAF-G469A Stable Cell Line - Ba/F3 | Inquiry |
| CSC-RT1742 | Human BRAF Knockout Cell Line-HeLa | Inquiry |
| CSC-RT2767 | Human BRAF Knockout Cell Line-HCT116 | Inquiry |
| CSC-RT2768 | Human BRAF Knockout Cell Line-DLD1 | Inquiry |
| CSC-SC001519 | Panoply™ Human BRAF Over-expressing Stable Cell Line | Inquiry |
| Cat.No. | Product Name | Price |
|---|---|---|
| CLKO-1589 | BRAF KO Cell Lysate-HeLa | Inquiry |
| CSC-DC001519 | Panoply™ Human BRAF Knockdown Stable Cell Line | Inquiry |
| CSC-RO0415 | Human BRAF-V600E Stable Cell Line - BaF3 | Inquiry |
| CSC-RO0732 | Human BRAF-K601N Stable Cell Line - Ba/F3 | Inquiry |
| CSC-RO0733 | Human BRAF-N486-P490del-K547Q Stable Cell Line - Ba/F3 | Inquiry |
| CSC-RO0734 | Human BRAF-V600_K601delinsE Stable Cell Line - Ba/F3 | Inquiry |
| CSC-RO0735 | Human BRAF-T599_V600insT Stable Cell Line - Ba/F3 | Inquiry |
| CSC-RO0768 | Human BRAF-G469A Stable Cell Line - Ba/F3 | Inquiry |
| CSC-RT1742 | Human BRAF Knockout Cell Line-HeLa | Inquiry |
| CSC-RT2767 | Human BRAF Knockout Cell Line-HCT116 | Inquiry |
| CSC-RT2768 | Human BRAF Knockout Cell Line-DLD1 | Inquiry |
| CSC-SC001519 | Panoply™ Human BRAF Over-expressing Stable Cell Line | Inquiry |
| Cat.No. | Product Name | Price |
|---|---|---|
| CLKO-1589 | BRAF KO Cell Lysate-HeLa | Inquiry |
| CSC-DC001519 | Panoply™ Human BRAF Knockdown Stable Cell Line | Inquiry |
| CSC-RO0415 | Human BRAF-V600E Stable Cell Line - BaF3 | Inquiry |
| CSC-RO0732 | Human BRAF-K601N Stable Cell Line - Ba/F3 | Inquiry |
| CSC-RO0733 | Human BRAF-N486-P490del-K547Q Stable Cell Line - Ba/F3 | Inquiry |
| CSC-RO0734 | Human BRAF-V600_K601delinsE Stable Cell Line - Ba/F3 | Inquiry |
| CSC-RO0735 | Human BRAF-T599_V600insT Stable Cell Line - Ba/F3 | Inquiry |
| CSC-RO0768 | Human BRAF-G469A Stable Cell Line - Ba/F3 | Inquiry |
| CSC-RT1742 | Human BRAF Knockout Cell Line-HeLa | Inquiry |
| CSC-RT2767 | Human BRAF Knockout Cell Line-HCT116 | Inquiry |
| CSC-RT2768 | Human BRAF Knockout Cell Line-DLD1 | Inquiry |
| CSC-SC001519 | Panoply™ Human BRAF Over-expressing Stable Cell Line | Inquiry |
Essential in the modulation of the MAP kinase/extracellular signal-regulated kinase (ERK) signaling pathway, which controls important cellular functions including proliferation, differentiation, and secretion, BRAF is a member of the RAF kinase family. Aberrations in this route have major effects on both normal physiological processes and pathological states, most especially cancer. Part of the three-tier MAPK/ERK signaling cascade, the BRAF gene found on chromosome 7q34 codes a serine/threonine protein kinase. Crucially important for signal transmission, this cascade gets dysregulated in many kinds of malignancies resulting from mutations in BRAF, which causes aberrant cellular growth and proliferation.
The BRAF gene has one of the most well-known mutations: glutamic acid (V600E) replaces valine at position 600. Driven by a constitutively active variant of the BRAF protein resulting from this particular mutation, continuous signal transduction throughout the MAPK/ERK pathway is accomplished without the requirement for outside stimuli including growth factors. Particularly in melanoma, the V600E mutation has been extensively investigated and identified as among the most often occurring cancer-causing mutations. Of melanomas, about 50–60% include BRAF mutations; V600E is the most often occurring variant. Beyond melanoma, BRAF mutations—including V600E and others—are also common in various other malignancies, including colorectal cancer, non-small cell lung carcinoma (NSCLC), thyroid carcinoma, and several kinds of leukemia, like hairy cell leukemia.
Being "driver" mutations—that is, essentially responsible for starting and maintaining tumorigenesis—what makes BRAF mutations especially important? This has made them a major emphasis in the field of focused treatments and precision medicine. For tumors including the V600E mutation, for instance, focused inhibition of BRAF with certain inhibitors like dabrafenib has demonstrated amazing results. However resistance usually shows up through different channels, hence combination usage of BRAF inhibitors with MEK inhibitors like trametinib has been used to postpone resistance and enhance clinical outcomes in BRAF-mutant malignancies.
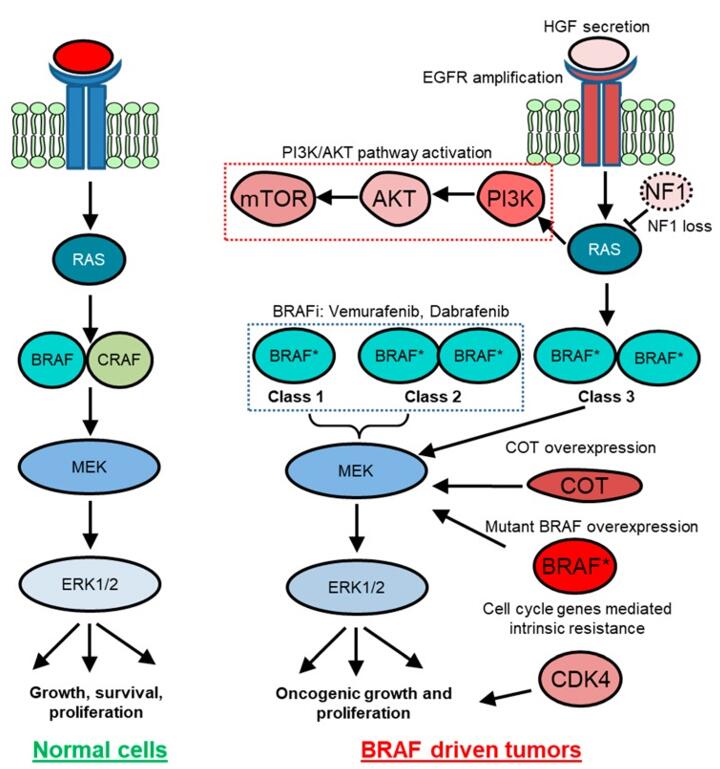 Figure 1. BRAF-mediated signaling in normal and cancer cells. (Zaman A, et al., 2019)
Figure 1. BRAF-mediated signaling in normal and cancer cells. (Zaman A, et al., 2019)
High-throughput genomic technology has revolutionized our understanding of cancer biology and made extensive tumor molecular profiling feasible. Among other large-scale projects, the Cancer Genome Atlas (TCGA) effort has been vital in compiling oncogenic mutations—including those in BRAF—across many cancer types. This not only clarifies the number and location of BRAF mutations but also helps to classify the molecular subtypes of cancers that predict response for treatment.
For instance, about 15% of colorectal tumors have BRAF mutations associated with a poor prognosis. But a BRAF mutation—especially the V600E variant—allows a target for precise therapy. Especially in papillary thyroid carcinoma, BRAF mutations are very widespread and a main diagnostic hint in thyroid tumors. Although less common (5–8%), BRAF mutations in NSCLC are a distinct genetic subtype of the cancer and are being treated with MAPK/ERK pathway-blocking drugs.
One of the significant challenges in targeting BRAF-mutant cancers is the development of resistance to therapy. Cancer cells are highly adaptive and often evolve mechanisms to bypass the inhibitory effects of targeted treatments. In the case of BRAF inhibitors, resistance can occur through several mechanisms:
Given the challenges caused by treatment resistance, combining strategies aiming at many sites in the signaling network has become a cornerstone of therapy for BRAF-mutant tumors. Combining BRAF and MEK inhibitors has shown promise in patients with BRAF V600E-mutant melanoma in extending progression-free survival above BRAF treatment alone. Targeting the major driver of tumor growth (BRAF) as well as its downstream effector (MEK) this dual inhibition strategy helps to lower resistance.
Researchers are also investigating combinations outside of the MAPK pathway. Combining BRAF/MEK inhibitors with immunotherapies including checkpoint inhibitors—e.g., anti-PD-1 or anti-CTLA-4 antibodies—is one area of active study. The rationale for this approach is that targeted inhibition of BRAF might raise tumor immunogenicity, hence raising their sensitivity to immune-based therapy.
Other fascinating strategies use novel drugs meant for RAF dimers or upstream activators like RTKs. Along with our understanding of the molecular foundations of BRAF-mutant tumors, the array of customized medicines aimed to overcome resistance and improve patient outcomes will grow alongside.
Although BRAF mutations are mostly linked with cancer, they also have a part in many non-malignant diseases. For uncommon genetic diseases like cardiofaciocutaneous syndrome, Noonan syndrome, and Costello syndrome, for instance, germline mutations in BRAF have been linked. BRAF mutations cause dysregulated MAPK/ERK signaling in several disorders marked by developmental delays, cardiac abnormalities, and unique facial traits.
These results underline the many roles BRAF plays in human life as well as the need for well-controlled MAPK signaling in preserving normal physiological processes. Whether by somatic mutations in cancer or germline mutations in genetic disorders, dysregulation of this pathway emphasizes the fundamental part BRAF plays in cell signaling and illness.
References:
If you don’t find the cell line you want, Creative Biogene can also provide stable cell line generation service with the best prices and fastest turnaround time for you! Contact us for more information or to request a quote.
Inquiry