Plasmids as Biologics
The rapid growth of the gene and cell therapy industry in recent years has greatly increased the demand for plasmid DNA. Plasmids are used to deliver genetic information or genes encoding therapeutic proteins, RNAs, or antigens directly to target cells in patients (Figure 1A). In addition, plasmids are used as vectors to deliver molecular components of gene editing systems (e.g., editing enzymes, RNA guides), including clustered regularly interspaced short palindromic repeats (CRISPR), zinc finger nucleases (ZFNs), and transcription activator-like effector nucleases (TALENs). In such in vivo uses, appropriate plasmids are combined with other components (e.g., adjuvants, lipids, etc.) to produce a pharmaceutical product that is delivered to patients. In these applications, plasmids are biotherapeutics and must be manufactured under current good manufacturing practices (cGMP) with appropriate regulation, testing, and controls.
Plasmids as Starting Materials
In addition to their role as biologics, plasmids play a supporting role as complex starting materials in the production of engineered cell products or other biologics (Figure 1). For example, in the context of chimeric antigen receptor T-cell (CAR-T) therapy, genome editing approaches, or mesenchymal stem cell therapy, plasmids are used as an alternative to viral vectors to genetically modify cells extracted from patients or donors. The first scenario requires transfection of the patient's T cells with a plasmid system (e.g., encoding the CAR gene, transposase, etc.) with the goal of achieving stable gene transfer, integration, and expression of the CAR. In the second scenario, plasmids are used to deliver the molecular components of gene editors such as CRISPR, TALEN, or ZFN. In either case, the plasmid-modified cells are infused back into the patient. Finally, plasmids are also used to modify mesenchymal stem cells, for example to enhance their therapeutic function in vivo.
Plasmids are also required for the production of viral vectors and mRNA, which can be used alone as biologics (Figure 1Bii) or as reagents for ex vivo modification of patient cells. For example, many adeno-associated viral (AAV) and lentiviral (LV) vectors are produced by transiently transfecting producer cells (e.g., human embryonic kidney (HEK) 293T cells) with multiple plasmids. For example, the production of AAV particles relies on the use of three different plasmids: an AAV transfer plasmid with the gene of interest flanked by two inverted terminal repeats (ITRs); a plasmid containing the AAV genes; and a helper plasmid encoding adenoviral helper genes. Similarly, the production of LVs by transient transfection of cells also requires the use of three or four different plasmids. The resulting viral vector can then be administered to a patient or used to transduce cells ex vivo.
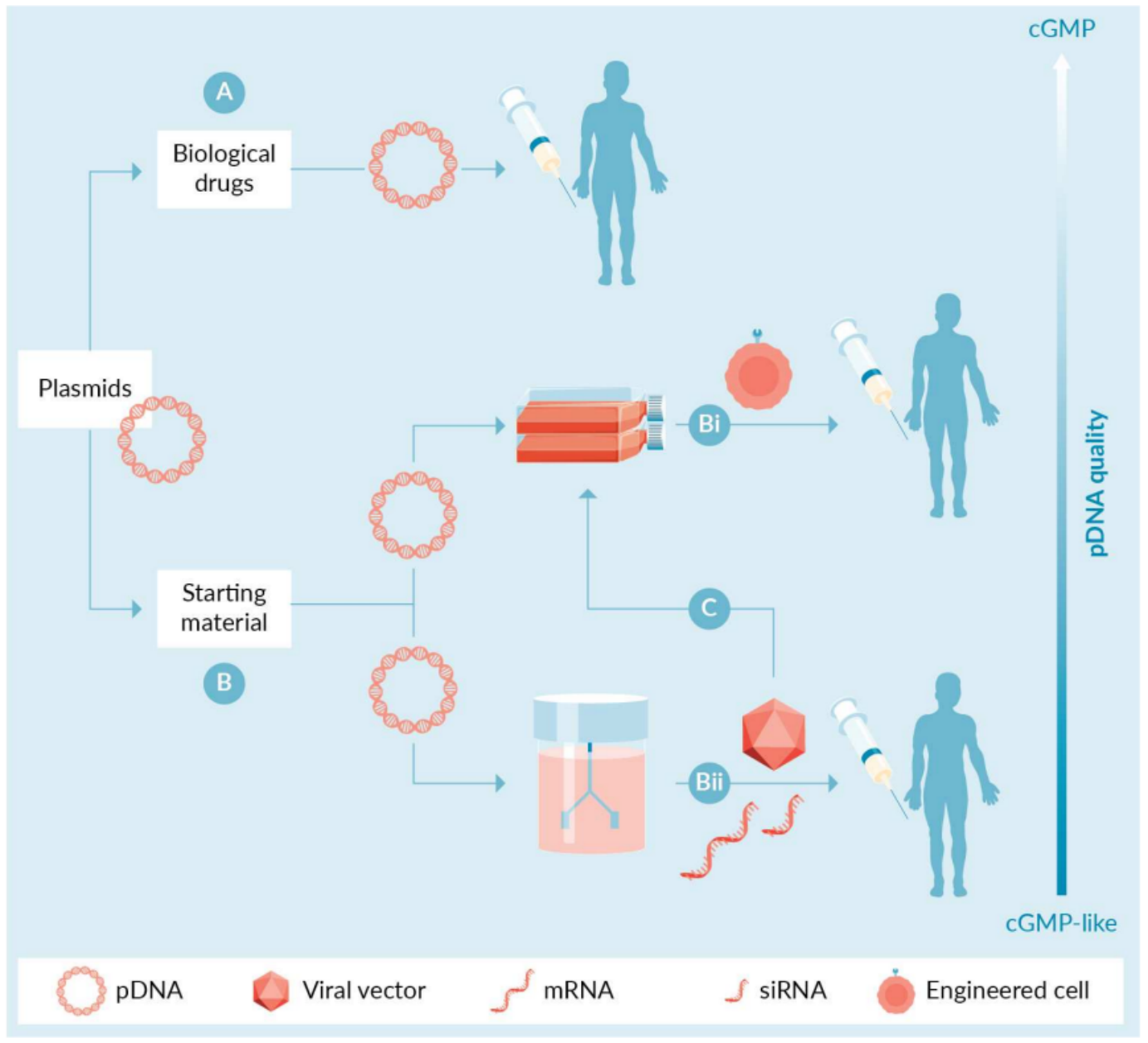
Figure 1. Direct and indirect applications of plasmid DNA in gene and cell therapies. (Prazeres D M., 2023)
Plasmid Grade
The indirect use of plasmid DNA as a starting material for viral vector or mRNA vaccine production requires the production of large quantities of material. For example, more than 1 kg of plasmid DNA is required to provide 1 billion doses of an mRNA vaccine. Since the plasmid will not appear in the final drug product that is directly administered to patients, but is used as a starting material for cGMP production of other starting materials, biopharmaceuticals or cell products, cGMP grade is not strictly required. However, although not all GMP aspects or GMP certificates are required, GMP principles should still be followed during the production process because the starting material may eventually remain in the finished drug product and may affect its quality, safety and efficacy. Ultimately, developers need to conduct an appropriate risk analysis to determine the quality standard of plasmid DNA that is suitable for further production of drug products under cGMP. Relevant aspects that need to be properly considered include quality management systems, documentation, raw materials, cell banks, production, specifications, testing, controls and storage. Therefore, one may choose to produce a cGMP-like/high-quality plasmid DNA that does not meet all cGMP requirements but still complies with many regulatory recommendations.
Large-Scale Plasmid Production
Despite its maturity, large-scale production of plasmid DNA is not an easy task, and producers are continually forced to find ways to increase productivity without compromising quality. This pressure to improve production performance stems in part from the fact that available production capacity is insufficient to respond in a timely manner to the demand growth associated with the development of growing plasmid applications.
Currently, large-scale production of plasmid DNA relies on only one platform host - E. coli. This preference is justified by the ability of E. coli to grow and divide rapidly and provide high plasmid DNA yields under a range of conditions. In addition, there are many tools that support molecular and microbial engineering of E. coli, including the creation of plasmid vectors and modified strains. Modified strains of E. coli can grow to densities of hundreds of grams per liter and produce up to 1-2 g of plasmid DNA per liter of culture.
One way to increase the amount of plasmid produced during production, simplify regulatory approval, and improve plasmid biological function is to focus on engineering the DNA backbone. There has been work to generate plasmids and plasmid systems (e.g., minicircles, nanoplasmids, microvectors) that are smaller, do not contain antibiotic resistance genes, increase yields, and provide high transgene expression.
Isolation and purification of plasmids from E. coli biomass recovered at the end of fermentation is an engineering challenge, but has been largely addressed, especially at smaller scales. The series of unit operations used in downstream processing of plasmids almost inevitably includes alkaline lysis, tangential flow filtration, and chromatography steps. Different combinations of operations are used to treat plasmid DNA with residual amounts of host impurities (genomic DNA, RNA, proteins, lipopolysaccharides, etc.) and meet regulatory requirements.
Once produced, large quantities of purified plasmid obtained from each batch should be rigorously characterized. Release specifications for plasmids used as starting materials will essentially focus on the same attributes as those covered when producing plasmids as biopharmaceuticals. This means that assays for identity (e.g., sequence, homogeneity), potency (e.g., concentration, homogeneity), and purity (e.g., host impurities, bioburden, residual kanamycin) must be developed, along with corresponding acceptance criteria.
| Cat.No. | Product Name | Price |
|---|---|---|
| RDQK-01 | Residual DNA Quantification Kit-E.coli | Inquiry |
| RDQK-02 | Residual DNA Quantification Kit-Pichia Pastoris | Inquiry |
| RDQK-03 | Residual DNA Quantification Kit- CHO | Inquiry |
| RDQK-04 | Residual DNA Quantification Kit- Vero | Inquiry |
| RDQK-05 | Residual DNA Quantification Kit - NS0 | Inquiry |
| RDQK-06 | Residual DNA Quantification Kit - MDCK | Inquiry |
| RDQK-07 | Residual DNA Sample Prep Kit | Inquiry |
| RDQK-08 | Residual DNA Size Analysis Kit-Vero | Inquiry |
| RDQK-09 | Residual DNA Quantification Kit for Plasmids | Inquiry |
| RDQK-09P | Residual DNA Quantification Kit for Plasmids Plus | Inquiry |
| RDQK-10 | Residual DNA Quantification Kit for human cell | Inquiry |
| RDQK-11 | Residual DNA Size Analysis Kit-Human cell | Inquiry |
| RDQK-11P | Residual DNA Size Analysis Kit-Human cell Plus | Inquiry |
Summary
The demand for large-scale production of plasmids has surged over the past few years, not only because of the development of plasmid biopharmaceuticals (e.g., for DNA vaccination, in vivo gene therapy, and gene editing), but primarily because of their supporting role in the production of many current gene and cell therapy products, including viral vectors, viral vector vaccines, mRNA vaccines, minicircles/microcarriers/nanoplasmids, and engineered cells. Looking ahead to plasmid production, several developments can be foreseen that will facilitate or completely change the way plasmids are produced today. For example, while the current performance of E. coli as a plasmid production host appears unmatched, one might wonder whether the high demand for plasmids justifies the search for a bacterial host with more suitable production characteristics. Gram-positive bacteria are advantageous as plasmid production hosts because they lack lipopolysaccharide, one of the most troublesome impurities when isolating plasmids from E. coli. Just as production hosts other than E. coli have emerged for recombinant protein production, there may be other hosts waiting to be discovered and developed as plasmid producers.
Reference
Prazeres D M. The supporting role of plasmids in gene & cell therapy. Cell Gene Ther. Insights, 2023, 9(05): 755-762.

