In the battlefield of cancer treatment, the RAS gene family has always been a fortress that is difficult to conquer. The KRAS and NRAS genes in the RAS gene family are like commanders of cell growth and division, regulating the normal growth of cells through signaling pathways. However, when these genes mutate, they become traitors, causing disordered cell proliferation and leading to cancer. Although scientists have developed targeted therapies for KRAS (G12C) and KRAS (G12D) mutations, NRAS-mutated cancers, especially NRAS (Q61*)-mutated melanomas, have always lacked effective treatment strategies. There are about 50,000 new cases of NRAS-mutated melanoma in the United States and Europe alone each year, which is undoubtedly a huge clinical demand gap.
Recently, in a research report entitled "Targeting the SHOC2–RAS interaction in RAS-mutant cancers" published in the international journal Nature, scientists from Novartis Institutes for BioMedical Research and other institutions published a breakthrough study revealing that SHOC2 protein may be a potential therapeutic target for NRAS-mutant cancers. This study not only brings new hope for the treatment of NRAS mutation cancers, but also injects new vitality into the field of cancer treatment.
The RAS gene family plays a key role in cell growth and division. Normally, RAS proteins regulate cell growth signals by binding to GDP (guanosine diphosphate) or GTP (guanosine triphosphate). However, when RAS genes mutate, these proteins are always activated, causing cells to proliferate uncontrollably, leading to cancer. In melanoma, mutations at the Q61 site of the NRAS gene are particularly common, accounting for about 20% to 30% of all melanoma cases. Although scientists have developed targeted therapies for KRAS mutations, treatments for NRAS mutations have been slow to develop.
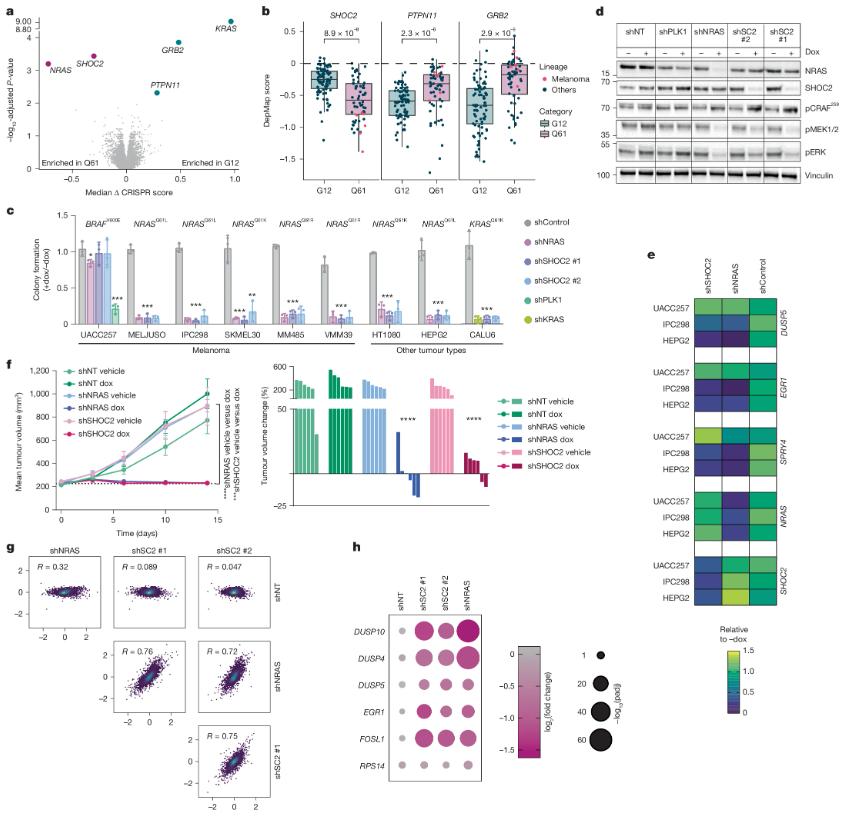
Figure 1. SHOC2 genetic validation in cancer models. (Hauseman Z J, et al., 2025)
Scientists used CRISPR gene editing technology to perform a genome-wide screening of Ba/F3 cell lines carrying KRAS and NRAS mutations, aiming to find gene dependencies associated with NRAS(Q61*) mutations. Through this approach, they found that the SHOC2 protein was significantly dependent in cells with NRAS(Q61*) mutations. Further experiments showed that sgRNA-mediated SHOC2 gene knockout significantly inhibited the growth of NRAS(Q61*) mutant cells, even more sensitive than KRAS(G12*) mutant cells. The researchers also used a melanoma xenograft model to further verify the therapeutic potential of SHOC2. The experimental results showed that the depletion of SHOC2 significantly inhibited the growth of NRAS(Q61*) mutant tumors, and its effect was comparable to NRAS gene knockdown, which suggests that targeting SHOC2 may block the MAPK signaling pathway in NRAS-driven melanoma.
| Cat.No. | Product Name | Price |
|---|---|---|
| CSC-RO0401 | Human KRAS-G12D Stable Cell Line - BaF3 | Inquiry |
| CSC-RO0402 | Human KRAS-G12C Stable Cell Line - BaF3 | Inquiry |
| CSC-RO0403 | Human KRAS-G12V Stable Cell Line - BaF3 | Inquiry |
| CSC-RO0404 | Human KRAS-Q61K Stable Cell Line - BaF3 | Inquiry |
| CSC-RO0405 | Human KRAS-G13D Stable Cell Line - BaF3 | Inquiry |
| CSC-RO0406 | Human KRAS-G13E Stable Cell Line - BaF3 | Inquiry |
| CSC-RO0407 | Human KRAS-G13R Stable Cell Line - BaF3 | Inquiry |
| CSC-RO0408 | Human KRAS-G13C Stable Cell Line - BaF3 | Inquiry |
| CSC-RO0593 | Human KRAS_G12C Stable Cell Line - NIH_3T3 | Inquiry |
| CSC-RO0594 | Human KRAS Stable Cell Line - NIH_3T3 | Inquiry |
| CSC-RO0744 | Human NRAS-Q61R Stable Cell Line - Ba/F3 | Inquiry |
| CSC-RO0745 | Human NRAS-Q61K Stable Cell Line - Ba/F3 | Inquiry |
| CSC-RO0746 | Human NRAS-G12C Stable Cell Line - Ba/F3 | Inquiry |
| CSC-RO0747 | Human NRAS-G12D Stable Cell Line - Ba/F3 | Inquiry |
| CSC-RO0748 | Human KRAS-G12C-H95D Stable Cell Line - Ba/F3 | Inquiry |
In this study, scientists not only discovered the direct interaction between SHOC2 and NRAS (Q61*) mutations, but also designed small molecule compounds that can bind to SHOC2 and disrupt SHOC2-RAS interactions through structural biology methods. These compounds showed dose-dependent inhibitory effects on the RAS/MAPK signaling pathway in in vitro experiments, providing strong evidence for the development of new therapeutic strategies. In addition, related studies also revealed the important role of SHOC2 in the RAS signaling pathway. By analyzing the interaction between SHOC2 and RAS protein, researchers found that this interaction is essential for maintaining the active state of RAS protein. This discovery not only provides new ideas for the treatment of NRAS mutant cancers, but also provides potential targets for the treatment of other RAS mutant cancers.
| Cat.No. | Product Name | Price |
|---|---|---|
| CSC-RO01267 | Human NRAS Stable Cell Line - BaF3 | Inquiry |
| CSC-RO0744 | Human NRAS-Q61R Stable Cell Line - Ba/F3 | Inquiry |
| CSC-RO0745 | Human NRAS-Q61K Stable Cell Line - Ba/F3 | Inquiry |
| CSC-RO0746 | Human NRAS-G12C Stable Cell Line - Ba/F3 | Inquiry |
| CSC-RO0747 | Human NRAS-G12D Stable Cell Line - Ba/F3 | Inquiry |
| CSC-RT0360 | NRAS Knockout Cell Line-293T | Inquiry |
| CSC-RT1672 | Human NRAS Knockout Cell Line-HeLa | Inquiry |
| CSC-SC010610 | Panoply™ Human NRAS Over-expressing Stable Cell Line | Inquiry |
Although this study has achieved encouraging results in the laboratory, there are still many challenges in translating these findings into clinical applications. First, these small molecule compounds need to be further optimized to improve their pharmacokinetic properties and bioavailability. Secondly, the efficacy and safety of these compounds need to be verified in more preclinical models. Finally, clinical trials are needed to evaluate the therapeutic effects of these compounds in patients with NRAS mutant cancers.
In summary, this study provides a new perspective for the treatment of NRAS-mutated cancers. By targeting SHOC2, researchers hope to develop a new treatment strategy to bring new hope to patients who currently lack effective treatment options.
Reference
Hauseman Z J, et al. Targeting the SHOC2–RAS interaction in RAS-mutant cancers. Nature, 2025: 1-10.

