Decades of research into chronic pain in humans have deepened scientists' understanding of the cellular mechanisms underlying this process. However, the inability to account for the biological variables underlying gender may limit the clinical application of these groundbreaking findings. Recently, in a research report titled "Sex differences in peripheral immune cell activation: Implications for pain and pain resolution" published in the international journal Brain, Behavior, and Immunity, scientists from the University of Alberta and other institutions have revealed differences in the way male and female mice develop and relieve chronic pain in their bodies. The findings point to potential ways for scientists to develop targeted therapies for use in humans in the future.
In the article, the researchers reported on their study of chronic pain in mice caused by inflammation rather than direct injury. They found that female mice were more sensitive to the effects of immune cells called macrophages, and they identified an X-linked receptor that is critical for resolving acute and chronic inflammation in both sexes. Professor Bradley Kerr said, "We are very interested in studying the causes of pain in the body, but in this study we went to the next step, which is to ask how pain is resolved to determine how these immune cells are involved."
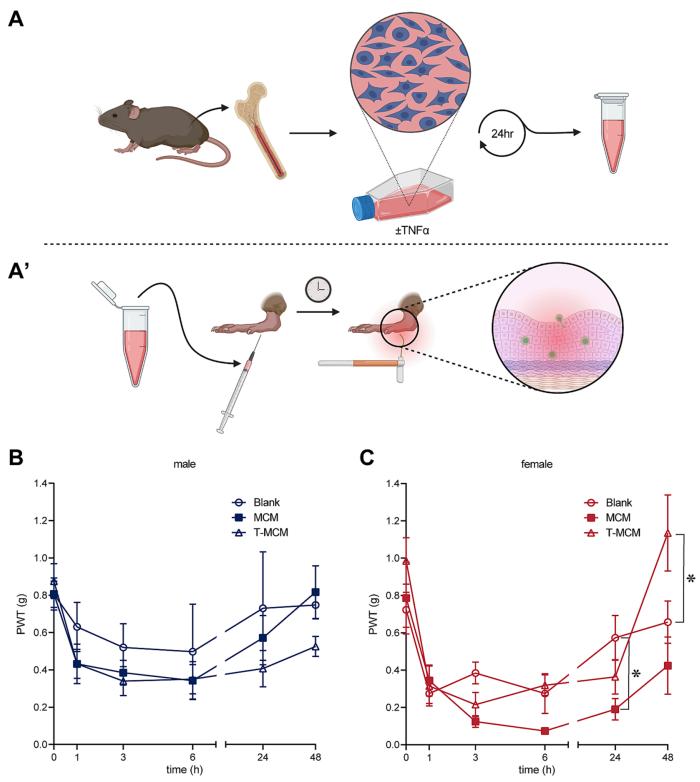
Figure 1. Female mice exhibit prolonged pain after hindpaw injection and TNFα dependant pain resolution. (Friedman T N, et al., 2023)
Our findings suggest that this may be down to the composition of the immune cells themselves, which affects not only the disease state but also whether the pain eventually progresses to chronic pain. According to Pain Canada, chronic pain is often defined as pain that lasts for three months or longer, or past the point of typical tissue healing. Approximately 20 per cent of Canadians suffer from chronic pain, with more women than men affected. Autoimmune diseases such as multiple sclerosis can cause chronic pain and are twice as common in women as in men.
Kerr points out that it was only in the past decade that scientists began using male and female mouse models in their pain studies, looking only for sex differences as a standardized research question. Kerr said his lab is very interested in studying the causes of chronic pain, and they hope to find effective ways to treat it. Pain early in the disease or after an injury is often protective. Researchers are interested in understanding pain that is no longer useful, but that doesn't guarantee safety or tell you that you should rest and let your injured leg heal. It's really important to understand where this pain comes from and how it goes away naturally, and now researchers may be one step closer to that goal.
Researchers used a variety of methods to analyze pain pathways in mouse models. Previously, researchers studying mice with multiple sclerosis found that female mice carried 2-3 times the number of pain receptors Tlr7 in their bodies than male mice. In this study, they genetically deleted the Tlr7 receptor and found that the pain in the mice was not properly resolved.
In contrast, the researchers treated mice with chronic pain using an antiviral drug used to treat warts, which already artificially stimulates Tlr7 expression. They found that compared with no treatment, the mice's body pain relief could be relieved 3-5 days earlier after treatment. Tlr7 is a special receptor in the body's immune system. When it detects a virus in the body, it activates an antiviral response, which is why you feel sore all over when you have a fever.
| Cat.No. | Product Nam | Pric |
|---|---|---|
| CSC-DC016006 | Panoply™ Human TLR7 Knockdown Stable Cell Line | Inquiry |
| CSC-SC016006 | Panoply™ Human TLR7 Over-expressing Stable Cell Line | Inquiry |
| AD16333Z | Human TLR7 adenoviral particles | Inquiry |
| LV00501Z | Human TLR7 lentiviral particles | Inquiry |
| CDCB162297 | Chicken TLR7 ORF Clone (NM_001011688) | Inquiry |
| CDCL186482 | Human TLR7 ORF clone(NM_016562.3) | Inquiry |
| CDCL186483 | Mouse TLR7 ORF clone(NM_133211.3) | Inquiry |
| CDFH019714 | Human TLR7 cDNA Clone(NM_016562.3) | Inquiry |
| CDFR005153 | Rat Tlr7 cDNA Clone(NM_001097582.1) | Inquiry |
| MiUTR1H-10438 | TLR7 miRNA 3'UTR clone | Inquiry |
| MiUTR1M-11797 | TLR7 miRNA 3'UTR clone | Inquiry |
Taken together, the results of this study indicate that although the cellular mechanisms of pain relief in the body may differ between genders, research on these differences is expected to help develop more targeted clinical application methods.
Reference
Friedman T N, et al. Sex differences in peripheral immune cell activation: Implications for pain and pain resolution. Brain, Behavior, and Immunity, 2023, 114: 80-93.