Recently, Cancer Discovery journal published the latest research results led by the research team of Fox Chase Cancer Center in the United States, which proposed a new strategy for transforming the immune microenvironment of "cold" tumors such as small cell lung cancers (SCLCs). With the help of screening based on CRISPR technology, the study found that inhibiting RNA helicase DHX9 can increase endogenous nucleic acids such as double-stranded RNA in cancer cells. Then a state similar to viral infection is created to activate "endogenous immune" mechanisms such as interferon response and successfully transform "cold" tumors.
"Creating a model similar to a virus infection" actually has a more concise professional term "Viral Mimicry", and the relevant mechanism was not well clarified until 2015. Simply put, under the influence and intervention of internal and external factors, such as the influence of epigenetic drugs (such as azacitidine, decitabine), endogenous retrotransposons in human cells may undergo abnormal transcription. Then endogenous DNA or RNA is produced, a typical representative is double-stranded RNA (dsRNA).
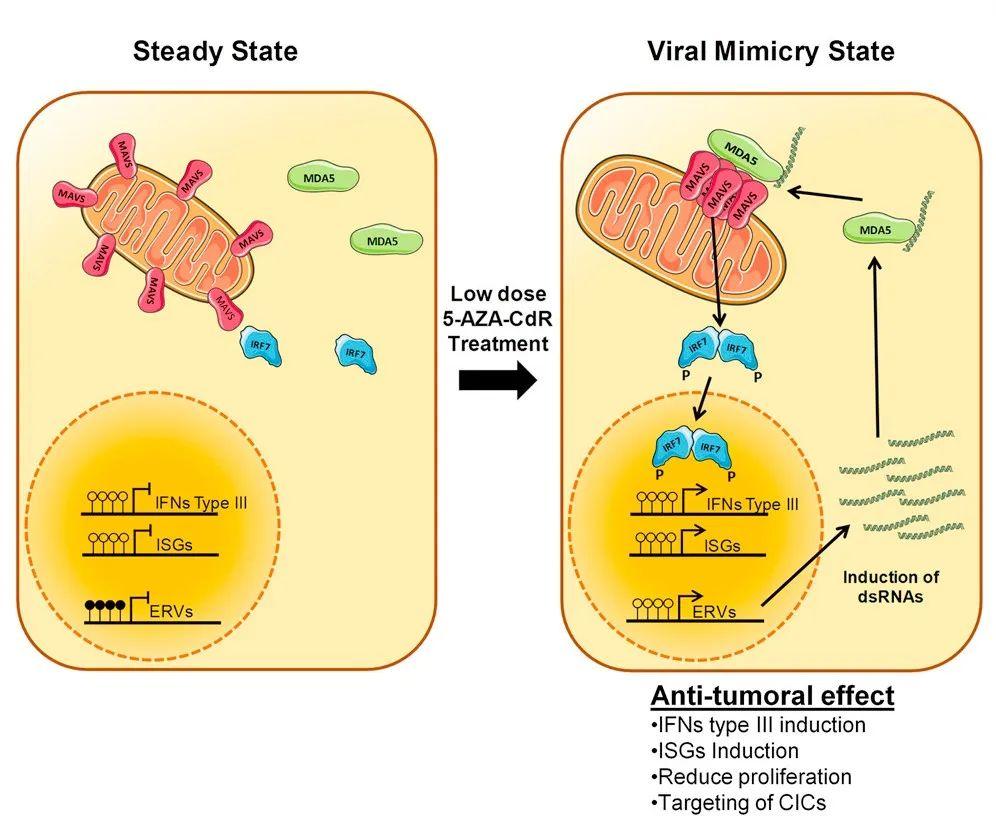
Figure 1. Schematic diagram of the mechanism by which epigenetic drugs trigger the phenomenon of "virus mimicry".
However, from the perspective of RNA sensors in human cells, the appearance of endogenous nucleic acids such as dsRNA signals that the cells have been infected by a virus. For example, endogenous retrovirus (ERV) infection will produce endogenous nucleic acids, and then RNA sensors will activate the anti-viral innate immunity and innate immune mechanisms in the human body, such as interferon response, which is very important for anti-viral and anti-tumor immunity. It is possible that the tumor immune microenvironment can turn from cold to hot, and immunotherapy can also be given free rein.
But the epigenetic factors that activate "viral mimicry" are not consistent across cancers. Therefore, researchers need to use CRISPR technology to screen out the activation switches in SCLC. The focus of the screening was on RNA helicase, and DHX9 quickly "stands out". And researchers found that SCLC is the cancer with the highest expression level of DHX9. High expression of DHX9 can be detected in most SCLC cell lines and is related to poor prognosis of patients.
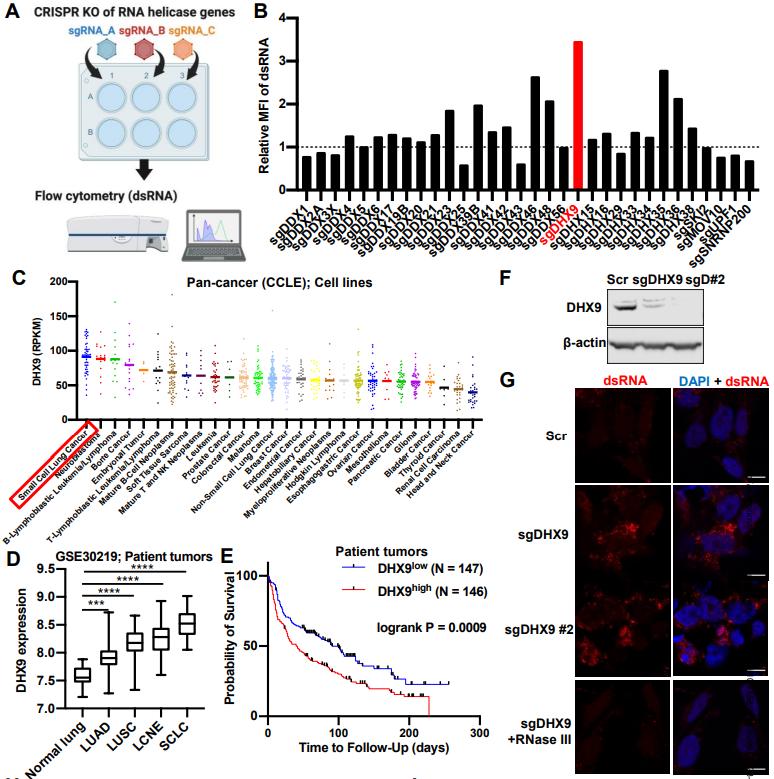
Figure 2. Screening process for DHX9 and its expression level in SCLC. (Murayama T, et al. 2024)
After DHX9 knockout in SCLC cell lines with small guide RNA (sgRNA), the researchers indeed observed significant accumulation of dsRNA. Under normal circumstances, DHX9 will effectively unwind them to prevent excessive levels of dsRNA in the cytoplasm. But after DHX9 knockout, no one will take care of the dsRNA, and even the accumulation of dsDNA will increase.
| Cat.No. | Product Name | Price |
|---|---|---|
| CSC-DC004299 | Panoply™ Human DHX9 Knockdown Stable Cell Line | Inquiry |
| CSC-SC004299 | Panoply™ Human DHX9 Over-expressing Stable Cell Line | Inquiry |
| AD04861Z | Human DHX9 adenoviral particles | Inquiry |
| LV10726L | human DHX9 (NM_001357) lentivirus particles | Inquiry |
| CDFR006909 | Rat Dhx9 cDNA Clone(NM_001107184.1) | Inquiry |
| MiUTR1M-03858 | DHX9 miRNA 3'UTR clone | Inquiry |
| MiUTR3H-05683 | DHX9 miRNA 3'UTR clone | Inquiry |
Further experiments immediately confirmed that DHX9 knockout was sufficient to upregulate the expression of numerous inflammation and immune response-related genes. Among them are a large number of interferon-stimulated genes (ISGs), as well as multiple innate immune markers of type I interferon response, indicating that the "virus mimicry" strategy has been successful. At the same time, researchers also detected that the expression of MHC class I molecules in SCLC cells increased significantly after DHX9 knockout, and even the expression of PD-L1, which is very rare in SCLC, was also significantly increased.
And DHX9 knockout has some "surprises." Since the expression of DNA damage-related genes is also up-regulated, the researchers further studied and found that DHX9 knockout will also make so-called "R-loops" (triple-stranded nucleic acid formed by hybridization of RNA strand and DNA template strand) unable to be unwinded and more likely to accumulate in cancer cells. This can not only aggravate the genomic instability of cancer cells, enhance the immunogenicity of cancer cells, but also activate the cGAS-STING pathway, both of which are beneficial to immunotherapy.
Finally, the researchers successfully reproduced the conclusions of previous cell experiments in mouse experiments, that is, DHX9 knockout in SCLC can effectively inhibit tumor growth, induce more immune cell infiltration (such as CD8 + T cells) and and powerful anti-tumor immune response. And DHX9 knockout can also synergize with immune checkpoint inhibitor treatment. In human SCLC patients, DHX9 expression levels are negatively correlated with immune infiltration, immune response, and patient survival prognosis.
Reference
Murayama T, et al. Targeting DHX9 triggers tumor-intrinsic interferon response and replication stress in Small Cell Lung Cancer. Cancer Discovery, 2024.