Cancer treatment has always been the focus of scientists around the world. From traditional surgery and radiotherapy to today's targeted therapy and immunotherapy, every technological advancement has brought new hope to patients. However, the complexity of cancer requires us to continue to explore the molecular mechanisms behind it.
Recently, in a research report entitled "Histone H1 deamidation facilitates chromatin relaxation for DNA repair" published in the international journal Nature, scientists from Shenzhen University School of Medicine and other institutions in China revealed the important role of histone H1 deamidation in DNA damage repair through research, which is expected to provide new ideas for cancer treatment.
DNA double-strand breaks (DSBs) are one of the most serious types of DNA damage, and their repair efficiency directly affects the genome stability and survival of cells.
As a connecting histone, histone H1 plays a key role in maintaining the higher-order structure of chromatin. However, how H1 modification regulates chromatin relaxation after DNA damage and thus affects DNA repair is still unclear to researchers. In this study, researchers aim to reveal the mechanism of action of H1 deamidation in DNA damage response and explore its potential application value in cancer treatment.
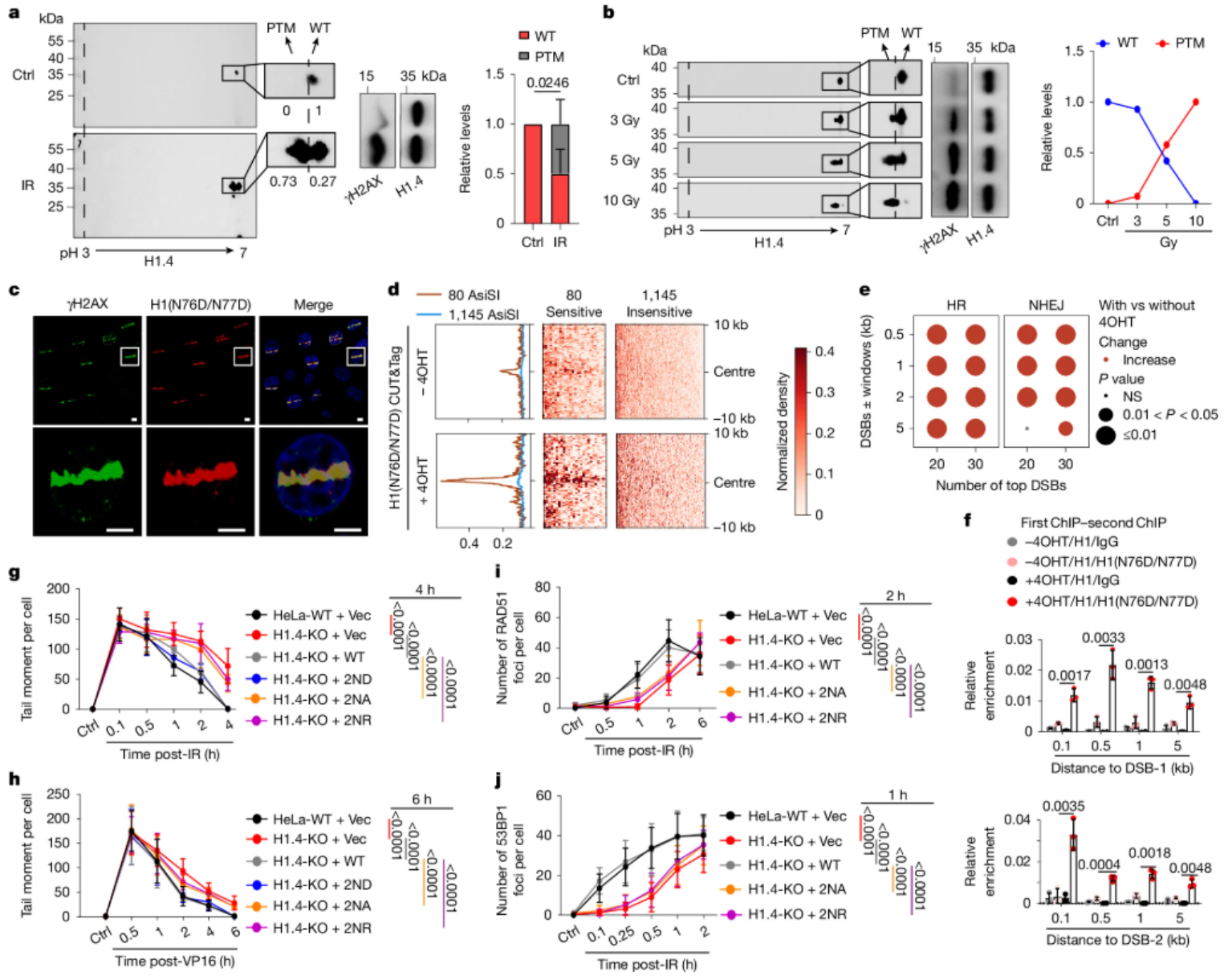
Figure 1. H1(N76D/N77D) is required for DNA damage repair. (Tian Y, et al., 2025)
In this study, the researchers mainly used the cervical cancer cell line HeLa cells as a model, and studied the role of H1 deamidation in DNA damage repair through a series of molecular biological techniques including mass spectrometry, immunoprecipitation, chromatin immunoprecipitation (ChIP) and gene editing. First, the researchers used two-dimensional gel electrophoresis (2DGE) technology to detect the modification changes of H1 after DNA damage. The results showed that asparagine residues 76 and 77 (Asn76 and Asn77) of H1 were deamidated after DNA damage. Further experiments showed that this deamidation was catalyzed by CTPS1 (cytosine triphosphate synthase 1) and was closely related to the acetylation of lysine residue 75 (Lys75) of H1. The researchers also knocked out CTPS1 or H1.4 by gene editing technology and introduced different H1.4 mutants in these cell lines to study their effects on DNA damage repair.
| Cat.No. | Product Name | Price |
|---|---|---|
| CSC-DC003808 | Panoply™ Human CTPS1 Knockdown Stable Cell Line | Inquiry |
| CSC-SC003808 | Panoply™ Human CTPS1 Over-expressing Stable Cell Line | Inquiry |
| MiUTR1H-02535 | CTPS1 miRNA 3'UTR clone | Inquiry |
The experimental results showed that H1 deamidation catalyzed by CTPS1 is a key step in chromatin relaxation after DNA damage. Deamidation of H1 not only promotes the binding of p300 to H1, but also creates conditions for subsequent H1K75 acetylation. This acetylation further promotes the relaxation of chromatin, which is conducive to the recruitment of DNA repair factors and the repair of DNA damage. In addition, the researchers also found that cells with high expression of CTPS1 showed higher tolerance to radiotherapy, which indicates that CTPS1 may be a potential target for cancer treatment.
This study revealed for the first time the important role of H1 deamidation in DNA damage repair and proposed a new molecular mechanism model. The relevant research findings not only enriched scientists' understanding of the dynamic regulation of chromatin, but also provided a theoretical basis for the development of new cancer treatment strategies. By regulating the activity of CTPS1 or directly targeting the H1 deamidation site, researchers in the future are expected to develop more effective cancer treatment drugs, thereby increasing patients' sensitivity to radiotherapy and chemotherapy. In summary, this study revealed the key role of histone H1 deamidation in DNA damage repair and pointed out that CTPS1 is a potential target for cancer treatment. This discovery is of great significance in the field of basic research and also brings new hope for clinical application.
Reference
Tian Y, et al. Histone H1 deamidation facilitates chromatin relaxation for DNA repair. Nature, 2025: 1-9.

