DNA methylation is an important epigenetic mark in mammals. Methylation of cytosines, primarily in the context of CpGs, ensures appropriate regulation of imprinted and tissue-specific genes, silences repetitive elements, and contributes to the function of key functional elements of the genome such as centromeres.
The DNA methylation patterns observed in mammalian tissues are the result of a dynamic process. First, most of the cytosine methylation brought by gametes is eliminated early in development, a process that includes passive demethylation and active demethylation by TET enzymes. Appropriate tissue- and cell-specific methyl marks are then reestablished in the embryo starting at implantation. This reestablishment of DNA methylation relies on methyltransferases, of which there are three species in mice but only two in humans: DNMT3A and DNMT3B.
Even after cells acquire an appropriate DNA methylation pattern, the overall stability of this pattern depends on the dynamic balance of gains and losses in cytosine methylation. In cell types expressing DNMT3A or DNMT3B, there may be a localized loss of DNA methylation due to TET activity, compensated by de novo DNA methylation.
| Cat.No. | Product Name | Price |
|---|---|---|
| CLKO-0268 | DNMT3B KO Cell Lysate-HeLa | Inquiry |
| CLKO-0733 | DNMT1 KO Cell Lysate-HEK293T | Inquiry |
| CLKO-1753 | DNMT3A KO Cell Lysate-HeLa | Inquiry |
| CSC-RT0111 | DNMT1 Knockout Cell Line-A549 | Inquiry |
| CSC-RT0470 | DNMT1 Knockout Cell Line-293T | Inquiry |
| CSC-RT1898 | DNMT3A Knockout Cell Line-HeLa | Inquiry |
| MiUTR1H-02940 | DNMT1 miRNA 3'UTR clone | Inquiry |
| MiUTR1H-02941 | DNMT3A miRNA 3'UTR clone | Inquiry |
| MiUTR1H-02942 | DNMT3B miRNA 3'UTR clone | Inquiry |
| SKO0153 | DNMT3B Validated sgRNA vector | Inquiry |
| SKO0434 | DNMT1 Validated sgRNA vector | Inquiry |
| AD05012Z | Human DNMT1 adenoviral particles | Inquiry |
| AD05013Z | Human DNMT3A adenoviral particles | Inquiry |
| AD05014Z | Human DNMT3B adenoviral particles | Inquiry |
| AD05015Z | Human DNMT3L adenoviral particles | Inquiry |
In addition, there is a global remodeling of DNA methylation during DNA replication. In fact, at this point, the two parental DNA strands carrying cytosine methylation are separated, and each is used as a template for the synthesis of the daughter strand, which is initially completely free of cytosine methylation.
Recently, researchers from the City University of Paris in France published an article titled "Non-canonical functions of UHRF1 maintain DNA methylation homeostasis in cancer cells" in the journal Nature Communications. This study reveals a non-canonical function of UHRF1 in maintaining DNA methylation homeostasis in cancer cells.
DNA methylation is an important epigenetic chromatin modification, and its maintenance in mammals requires the protein UHRF1. It is unclear whether UHRF1 acts solely by stimulating DNMT1 to maintain DNA methylation or whether it has important additional functions.
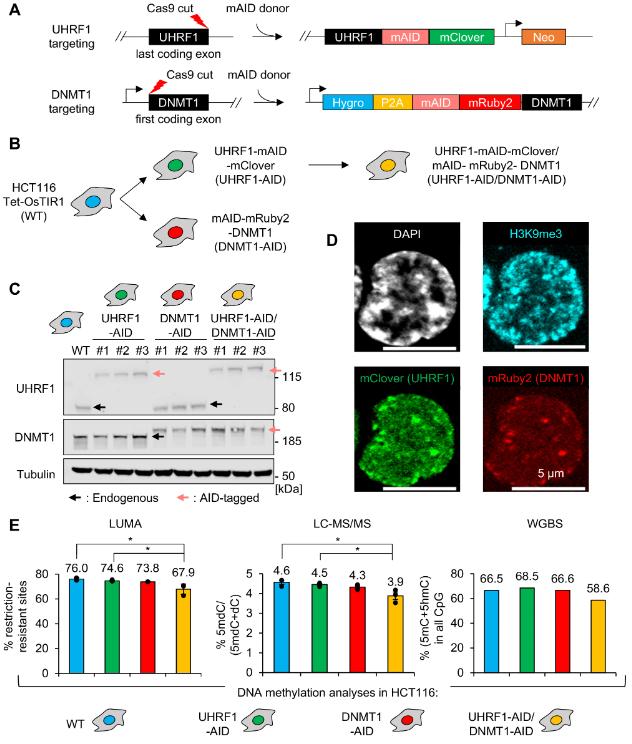
Figure 1. Establishment and validation of endogenous AID-tagged UHRF1 and/or DNMT1 HCT116 cells. (Yamaguchi K, et al. 2024)
Using degranulation alleles, the researchers found that UHRF1 deletion resulted in a much greater loss of DNA methylation than DNMT1 deletion. This is not caused by passive demethylation, as UHRF1-deficient cells proliferate more slowly than Dnmt1-deficient cells. In contrast, bioinformatics, proteomics, and genetic experiments confirmed that UHRF1, in addition to activating DNMT1, interacts with and promotes the activity of DNMT3A and DNMT3B.
In addition, the researchers found that UHRF1 can antagonize TET2-activated DNA demethylation. Therefore, UHRF1 has a non-canonical role and contributes significantly to DNA methylation homeostasis. These findings have practical implications for epigenetics in health and disease.
Cancer cells have abnormal epigenomes, which creates opportunities for anti-tumor treatments. Among various epigenetic marks, DNA methylation has proven to be a valuable target. The DNMT1 inhibitor 5-aza-cytidine has been successfully used in the clinical treatment of myeloid dysplasia and acute myeloid leukemia, but it has limitations such as high toxicity, rapid degradation and emergence of drug resistance.
A new generation of selective DNMT1 inhibitors may alleviate these problems, but these molecules still trigger DNMT1 degradation, which may have unintended side effects. The data here indicate that a completely different strategy may be feasible, by targeting UHRF1 instead of DNMT1, justifying ongoing drug design efforts.
As with any essential protein, one of the challenges will be identifying a therapeutic dosage window and/or an appropriate delivery method that damages cancer cells without damaging healthy cells. Taken together, this work reveals a non-canonical function of UHRF1 and opens the way for further exploration of the role of this key epigenetic regulator in normal cells and disease.
Reference
Yamaguchi K, et al. Non-canonical functions of UHRF1 maintain DNA methylation homeostasis in cancer cells. Nature Communications, 2024, 15(1): 2960.

