Research On Glial Cells
Neurological damage or degenerative diseases, such as Alzheimer's disease, Parkinson's disease, macular degeneration, etc., often lead to the loss of neurons and damage to neural circuits. Unlike species with strong self-renewal ability, mammals have almost lost the ability to regenerate neurons in the central nervous system after adulthood. Therefore, the above symptoms can cause a large number of neuronal deaths, leading to irreversible and severe functional damage. Glial cells have been reported to have certain plasticity. In recent years, reprogramming glial cells in vivo and transforming them into neurons has become a hot research field and has the potential to become a new therapeutic strategy. These glial cells include astrocyte and microglia in the brain, Müller glia in the retina or NG2 glia in the spinal cord. Researchers often use viral tools (such as adeno-associated virus or Lentivirus) to manipulate the expression of one or several transforming factors in glial cells, and induce in situ regeneration of neurons in adults. These factors often play a key role in the differentiation of neural stem cells, progenitor cells, or precursor cells during neural development, and some are even considered as cell fate determinants.
Debate on the Role of NeuroD1 in Transdifferentiation
Among them, the role of NeuroD1 in the process of transdifferentiation has been widely studied and fiercely debated. As early as 2014, Professor Chen Gong's team from Jinan University of China reported that NeuroD1 was delivered to astrocyte through a virus tool using the "glial cell specific" GFAP micro promoter to express NeuroD1, which can achieve ultra efficient in situ regeneration of neurons in the brain of Alzheimer's disease mouse models. However, a study published in Cell in 2021 questioned the authenticity of NeuroD1's transdifferentiation. They found that the "regenerative neurons" found in previous studies did not originate from astrocyte, but only from endogenous neurons mislabeled due to AAV leakage expression caused by GFAP micro promoter. Surprisingly, when using GFAP micro promoters to express certain other transdifferentiation factors, this type of leakage expression also exists to some extent and exhibits irregularity. After that, Chen Gong's team questioned the viral titer used by Zhang Chunli's team in the cell paper, and believed that the reason for the false labeling was that the latter used a virus with a high titer (1013 GC/mL) to infect astrocyte, which would increase the risk of cytotoxicity and would inevitably lead to the non-specific expression of NeuroD1 in endogenous nerve cells. So far, there is a lack of clear consensus on this debate in this research field. Moreover, if the existing glial cell AAV delivery system is unable to avoid leakage expression in endogenous neurons, it is urgent to develop a new AAV system for highly specific infection of glial cells.
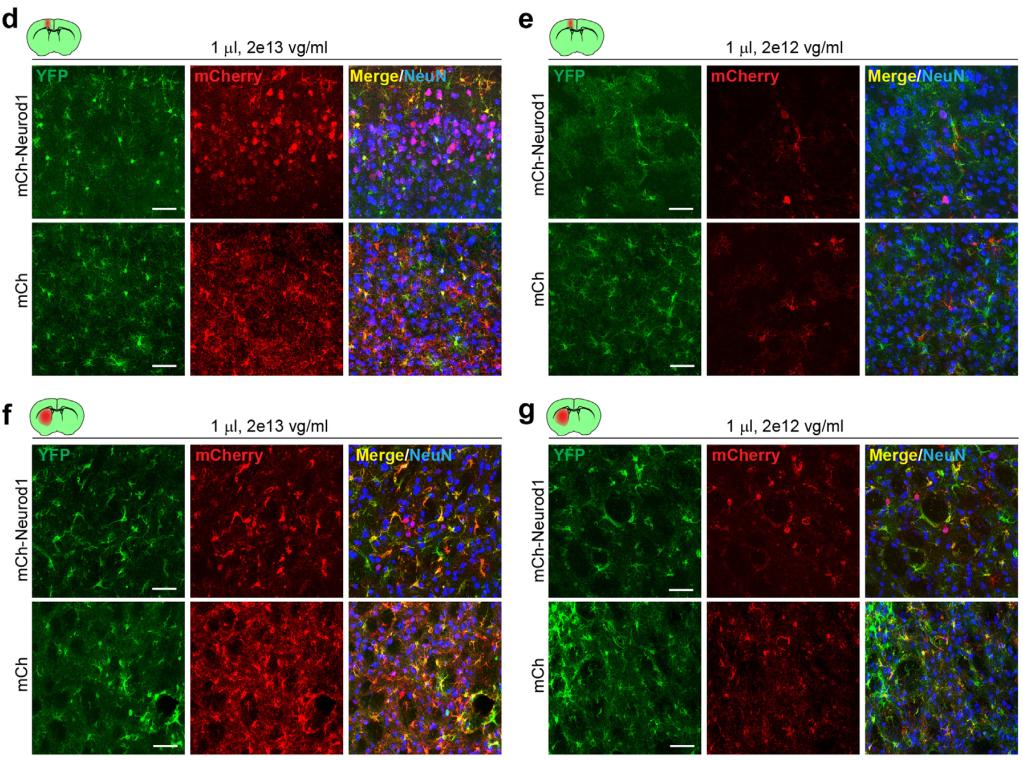
AAV-GFAP-mediated Neuronal Leakage Is Transgene-dependent
Professor Chen Bo's team from the Department of Ophthalmology and Neuroscience at the Icahn School of Medicine in Mount Sinai, New York, USA, published a research paper titled "New AAV tools failed to detect Neurod1-mediated neurological conversion of Müller glia and astrocytes in vivo" in the journal eBioMedicine. In this paper, the author used strict genetic lineage tracing (specifically labeled Müller glial cells) and delivered four widely studied reprogramming factors, NeuroD1, Math5, ASCL1, or NeuroG2, to Müller glial cells in the retina of adult mice one by one using GFAP micro promoters, providing in-depth and systematic exploration of the above debate. The experimental results indicate that the GFAP micro promoter mediated AAV leakage expression is not caused by high viral titer, but is determined by the transformation factor it carries and expresses. On the contrary, the use of low viral titers (1011-1012GC/mL) cannot eliminate leakage expression, but can lead to a sharp decrease in infection efficiency. When using different serotypes of AAV to infect Müller glial cells through different injection routes, leakage expression cannot be eliminated, but the type of neurons that leak expression can be changed. More interestingly, when the author uses two modified new AAV systems to introduce these four reprogramming factors into the retina, it can greatly reduce or even eliminate leakage expression. The results of the new AAV system further indicate that even after achieving highly specific expression in Müller glial cells, a single transforming factor such as NeuroD1 cannot achieve glial cell differentiation into neurons. Finally, when the new AAV system was used to test astrocyte in different regions of the brain, the authors also did not detect NeuroD1 mediated transdifferentiation.
Figure 1. Lowering AAV doses does not correct neuronal leakage after AAV-GFAP-mediated delivery of Neurod1, Math5, Ascl1, and Neurog2. (Xie Y, et al., 2023)
AAV Vectors and Production Method
In order to construct a double-gene AAV-GFAP vector, the coding sequences of Neurod1 (mouse), Math5 (mouse), Ascl1 (mouse), Neurog2 (human), GFP, and mCherry with P2A self-cleavage sites were subcloned into the AAV vector backbone driven by a GFAP promoter. For the AAV-CAG-FLEX vector, the protein-coding region of AAV-CAG-FLEX-GFP is replaced by mCherry or TF-P2A-mCherry digested from the AAV-GFAP vector. In order to construct a triple-gene AAV vector, the coding sequences of mCherry, GFP, BFP, and Ascl1 with P2A and T2A self-cleavage sites were subcloned into the AAV-GFAP vector backbone. All vectors were validated by restriction enzyme digestions and DNA sequencing. For AAV production, AAV vectors, rep cap plasmids ShH1029, PHP.eB or AAV5, and AAV helper plasmids are used for co-transfection in AAVpro 293T cell lines.
Conclusion
This study clarifies that the reporter genes on viral tools only indicate their current expression levels and cannot be simply used to trace cell sources. The specific expression of control reporter genes such as GFP, mCherry, etc. is not enough to show that the virus tool will maintain high specificity of glial cells when carrying other Reprogramming factors. The use of lineage tracking technology is the "gold standard" for testing whether glial cells truly differentiate into regenerative neurons. The key to the study of glial Reprogramming is to express related factors in glial cells closely tracked by lineage. In addition, the conclusion that lineage-tracking mice may hinder Neurod1 mediated transdifferentiation proposed by Chen Gong's team previously, the author said in the discussion of the article that, so far, several research teams have used the pedigree tracking technology to realize the reprogramming of glial cells into regenerative neurons in different regions or tissues such as the brain, retina, and spinal cord. Therefore, it is not safe to conclude that lineage tracking of mice hinders transdifferentiation.
References
1.Xie Y, et al. New AAV tools fail to detect Neurod1-mediated neuronal conversion of Müller glia and astrocytes in vivo. Ebiomedicine, 2023, 90.
2.Guo Z, et al. In vivo direct reprogramming of reactive glial cells into functional neurons after brain injury and in an Alzheimer’s disease model. Cell stem cell, 2014, 14(2): 188-202.
3.Wang L L, et al. Revisiting astrocyte to neuron conversion with lineage tracing in vivo. Cell, 2021, 184(21): 5465-5481. e16.