Professor Michael Elowitz of California Institute of Technology and others published a research paper titled "Synthetic protein circuits for programmable control of mammalian cell death" in the top international academic journal Cell. In this study, the research team developed a synthetic protein-level cell death circuit - the Synpoptosis circuit, which regulates cell death execution proteins through hydrolysis, thereby controlling mammalian cell apoptosis and pyroptosis. Furthermore, this circuit can be delivered and passed between cells using virus-like particles (VLPs), providing the basis for engineering synthetic killer cells that induce desired death programs in target cells without self-destruction. Taken together, these results lay the foundation for programmable control of mammalian cell death.
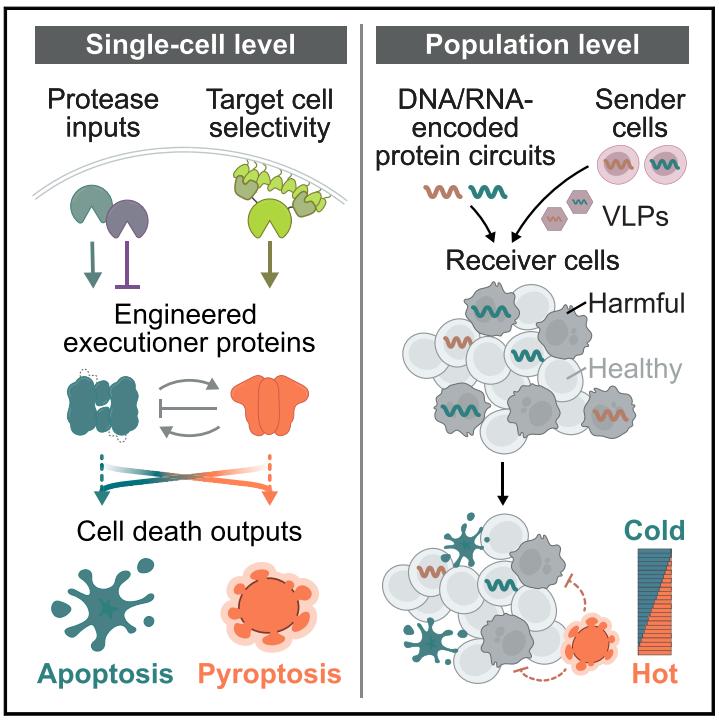
Figure 1. Naturally inspired protein engineering produces synpoptosis circuits that programmably control user-selectable death programs in target mammalian cells. (Xia S, et al., 2024)
Mammalian systems, including humans, use different cell death programs to eliminate harmful cells and develop immunity. Apoptosis is immunologically "cold". In contrast, pyroptosis is immunologically "hot" and involves the massive release of damage-associated molecular patterns (DAMPs).
Apoptosis and pyroptosis each have advantages depending on the immune context. The immunostimulatory properties of pyroptosis can promote cell killing. For example, inducing pyroptosis in a minority of 15% of cells is sufficient to clear the entire tumor by enhancing antitumor immunity. Consistent with this, the expression of the gasdermin (GSDM) protein family, the executor of pyroptosis, is positively correlated with the survival of cancer patients, and cytotoxic lymphocytes (CTL) upregulate the expression of GSDM in cancer cells. To escape pyroptosis, cancer cells produce inactivating GSDM mutations, silence GSDM expression, and express non-pyroptotic GSDM variants. However, if pyroptosis is excessively triggered, it can lead to pathological inflammation. Therefore, it is desirable to controllably induce apoptosis or pyroptosis and modulate their relative frequency.
Existing cell killing methods cannot fully guide the manner of cell death. Cytotoxic drugs are generally limited to triggering apoptosis in cold tumors. CAR-T cells can effectively target cells expressing a single antigen or multiple antigens. However, in order to kill target cells, CAR-T cells usually use granzymes, and granzymes may induce apoptosis or pyroptosis. Other studies have attempted granzyme-independent approaches, including engineering TRAIL-presenting cells and regulating synthetic circuits of caspases, BID, or BAX. However, these methods are also limited to inducing apoptosis.
To achieve customized control of cell death, a set of synthetic circuits with the following characteristics is required:
1. These circuits should allow activation and inhibition of apoptosis and pyroptosis.
2. They should direct cell death patterns in various cellular environments.
3. They should allow the integration and calculation of multiple input signals.
4. They should be able to selectively kill target cells.
5. They should support cell-to-cell spread, making it possible to engineer synthetic killer cells to eliminate other cells with designed death programs.
In this study, the research team proposed a synthetic protein-level cell death circuit with the above characteristics-Synpoptosis circuit. To engineer these circuits, the research team drew inspiration from natural cell death pathways that utilize mechanisms that regulate proteolysis as well as protein-level caging and degradation. Synpoptosis circuits provide the basis for rationally designed, programmable control of mammalian cell death.
Specifically, apoptosis-related caspases promote apoptosis, and they can also punch holes in pyroptosis by cleaving the pyroptosis protein GSDM. Tobacco etch virus protease (TEVP) can activate modified caspase-3. To achieve more complex downstream apoptosis control functions, the research team designed additional caspase-3 variants that can modulate its apoptotic activity through TEVP cleavage. Next, the research team designed the regulation of cell pyroptosis by inserting the cleavage sites of TEVP, tobacco vein mottling virus protease (TVMVP), and hepatitis C virus protease (HCVP) into the connecting region between the N-terminal and C-terminal domains of 3 mammalian GSDMs. Each engineered GSDM effectively triggered pyroptosis in the presence of cognate proteases. The research team focused on GSDMA in subsequent experiments. Like caspase-3, the research team designed additional GSDM variants that can exert both positive and negative control of pyroptosis. This synthetic protein circuit can regulate apoptosis and pyroptosis in both directions. Next, the research team used this protein circuit to cause pyroptosis in cells susceptible to apoptosis, causing pyroptosis-prone cells to undergo apoptosis to adjust the ratio between apoptosis and pyroptosis in the cell death mode in various cellular environments.
| Cat.No. | Product Name | Price |
|---|---|---|
| CSC-DC002394 | Panoply™ Human CASP3 Knockdown Stable Cell Line | Inquiry |
| CSC-RK0260 | Human CASP3 Knockdown Cell Line-HeLa | Inquiry |
| CSC-RT0524 | Human CASP3 Knockout Cell Line-HeLa | Inquiry |
| CSC-SC002394 | Panoply™ Human CASP3 Over-expressing Stable Cell Line | Inquiry |
| AD00068Z | Human CASP3 adenoviral particles | Inquiry |
| AD00069Z | Sus scrofa CASP3 adenoviral particles | Inquiry |
| AD03011Z | Human CASP3 adenoviral particles | Inquiry |
| LV07957L | human CASP3 (NM_032991) lentivirus particles | Inquiry |
| CLKO-0029 | CASP3 KO Cell Lysate-HeLa | Inquiry |
Mammalian systems trigger specific cell death programs based on cell state and desired immune outcome. Likewise, many therapeutic challenges could be solved if the right cells could be killed in the right way. For example, Synpoptosis circuits can be delivered to tumors to conditionally induce cancer cell death in a manner that safely enhances anti-tumor immunity. This circuit can also be used to eliminate cells that play other harmful roles, such as senescent cells, fibrotic cells, autoimmune cells, and infected cells, and can do this with appropriate immune stimulation.
Reference
Xia S, et al. Synthetic protein circuits for programmable control of mammalian cell death. Cell, 2024.

