Recently, researchers published a research paper titled "Cross-species tropism of AAV.CPP.16 in the respiratory tract and its gene therapies against pulmonary fibrosis and viral infection" in Cell Reports Medicine, a subsidiary of Cell. The study showed that AAV.CPP.16 has a high tropism for respiratory tissues across species (including mice and non-human primates), and verified the gene supplementation and gene editing therapy delivered by nasal administration of AAV.CPP.16 for the treatment of idiopathic pulmonary fibrosis and viral infection. This study shows that AAV.CPP.16 is a promising vector for gene therapy and gene editing in the respiratory system and lungs.
Efficient gene delivery vectors are essential for the treatment of respiratory and lung diseases. In this latest study, the research team confirmed that AAV.CPP.16, an engineered AAV variant derived from AAV9, can effectively transduce airway and lung cells in mice and non-human primates through intranasal administration.
| Cat.No. | Product Name | Price |
|---|---|---|
| VLP-AAV006 | AAV9 Virus-Like Particles (Empty Capsids) | Inquiry |
| AAV00274Z | AAV9-cTNT-GFP | Inquiry |
| AAV00286Z | AAV9-CMV-RLuc | Inquiry |
| AAV00534Z | AAV9-hSyn-NULL | Inquiry |
| AAV00119Z | AAV9-CMV-Luc | Inquiry |
| AAV00271Z | AAV9-Syn-iCre | Inquiry |
| AAV00278Z | AAV9-CAG-GCaMP6m | Inquiry |
| AAV00289Z | AAV9-EF1α-mCherry | Inquiry |
| AAV00290Z | AAV9-CAG-Gluc | Inquiry |
| AAV00294Z | AAV9-Syn-RFP | Inquiry |
| AAV00405Z | scAAV9-CAG-GFP | Inquiry |
| AAV00258Z | AAV9-CAG-Cre | Inquiry |
| AAV00259Z | AAV9-CAG-Cre-GFP | Inquiry |
| AAV00285Z | AAV9-tMCK-mCherry | Inquiry |
| AAV00532Z | AAV9-CAG-Cre-2A-GFP | Inquiry |
AAV.CPP.16 outperformed AAV6 and AAV9, two wild-type AAVs that have been shown to have tropism for respiratory tissues, and can efficiently target key respiratory cell types.
Next, the research team validated gene supplementation and gene editing therapies delivered by AAV.CPP.16 in two clinically relevant mouse models of respiratory and lung diseases. The results showed that a single intranasal administration of AAV.CPP.16 expressing a dual-target VEGF/TGF-β1 neutralizing protein could protect the lungs from idiopathic pulmonary fibrosis. A single intranasal administration of AAV.CPP.16 carrying the "all-in-one" CRISPR-CasRx RNA editing system can inhibit the transcription of the RNA-dependent RNA polymerase (Rdrp) gene derived from SARS-CoV-2.
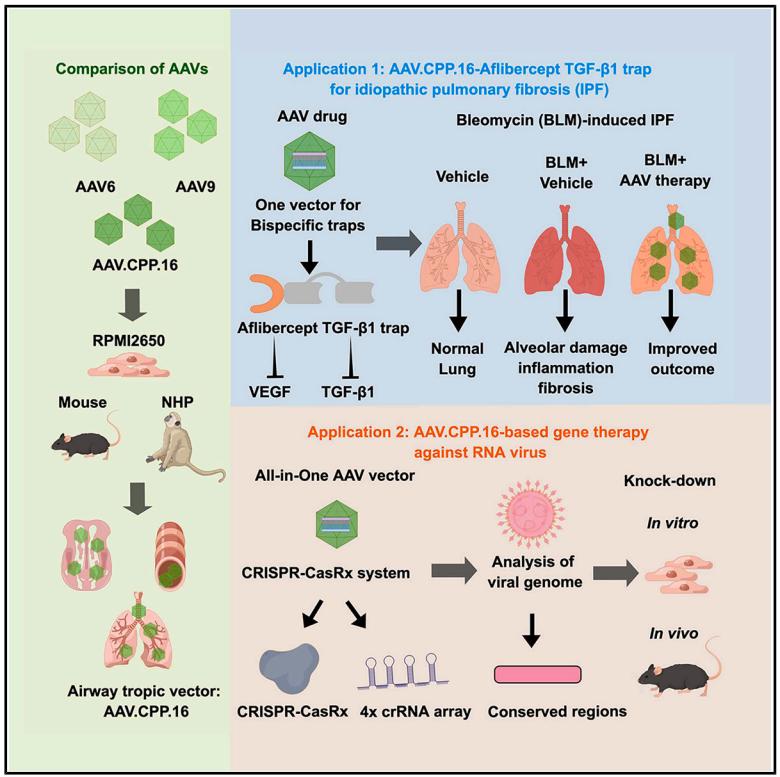
Figure 1. AAV.CPP.16 efficiently transduces airway and lung cells in rodents and primates. (Yang Z, et al., 2025)
In summary, this study introduces AAV.CPP.16, an intranasal gene delivery vector that is highly tropistic for respiratory tissues in mice and non-human primates. The research team further validated two AAV.CPP.16-mediated gene therapies: one for the treatment of idiopathic pulmonary fibrosis by expressing a bifunctional fusion protein that inhibits VEGF and TGF-β1; the other is a passive therapy for preventing SARS-CoV-2 infection using the CRISPR-CasRx system. These results suggest that AAV.CPP.16 is a promising vector for respiratory and lung gene therapy and gene editing.
Reference
Yang Z, et al. Cross-species tropism of AAV. CPP. 16 in the respiratory tract and its gene therapies against pulmonary fibrosis and viral infection. Cell Reports Medicine, 2025.

