• Adenovirus Service • AAV Service • Lentivirus Service • Retrovirus Service


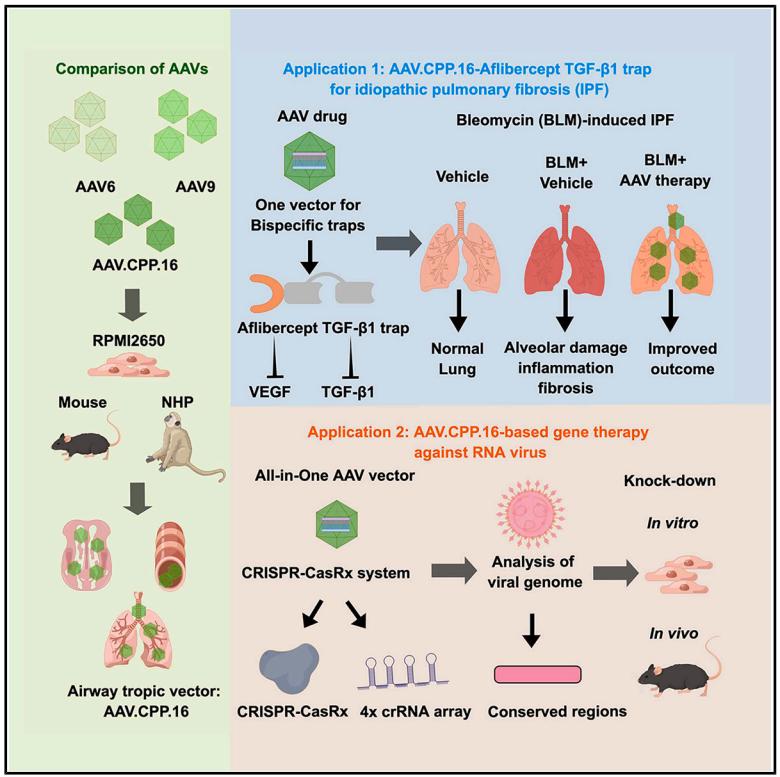
Recently, researchers published a research paper titled "Cross-species tropism of AAV.CPP.16 in the respiratory tract and its gene therapies against pulmonary fibrosis and viral infection" in Cell Reports Medicine, a subsidiary of Cell. The study showed that AAV.CPP.16 has a high tropism for respiratory tissues across species (including mice and non-human primates), and verified the gene supplementation and gene editing therapy delivered by nasal administration of AAV.CPP.16 for the treatment of idiopathic pulmonary fibrosis and viral infection. This study shows that AAV.CPP.16 is a promising vector for gene therapy and gene editing in the respiratory system and lungs.
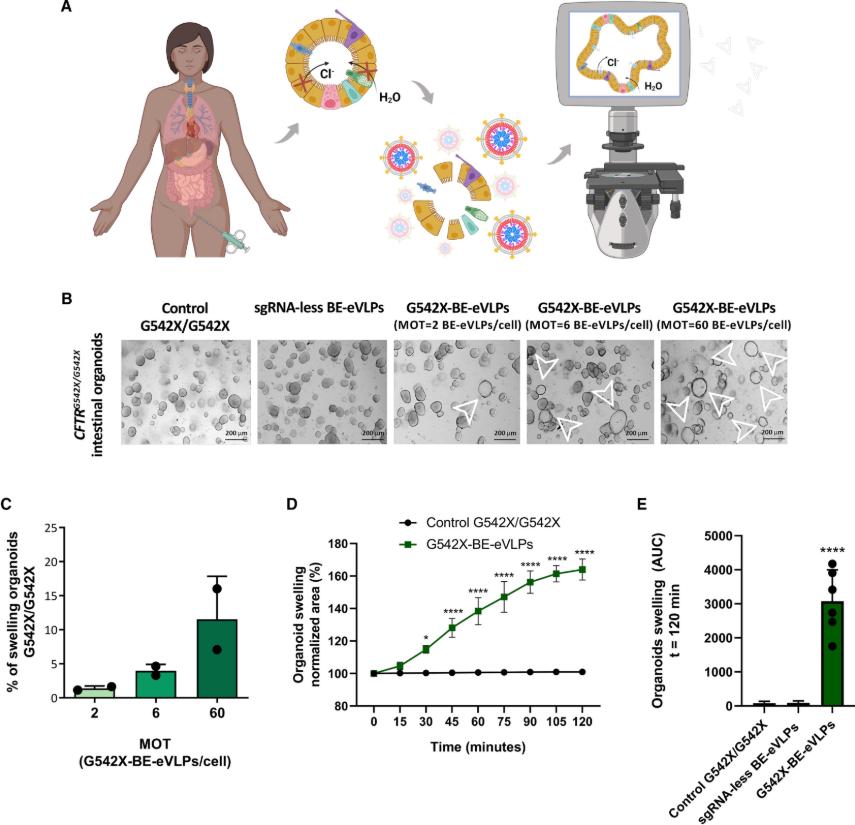
Around the world, about 162,000 people are struggling with cystic fibrosis (CF), a serious genetic disease. CF is an autosomal recessive genetic disease that severely shortens the lifespan of patients. It is caused by loss-of-function mutations in the CF transmembrane conductance regulator (CFTR) gene. These mutations affect the synthesis, folding, transport, and gating properties of the CFTR anion channel, thereby interfering with the transport of chloride and bicarbonate ions. Although modulatory drugs have made some progress in the treatment of CF, existing modulatory drugs are powerless for patients carrying specific mutations, such as those with the G542X mutation. Recently, a study published in iScience titled "Adenine base editing with engineered virus-like particles rescues the CFTR mutation G542X in patient-derived intestinal organoids" has brought new hope.
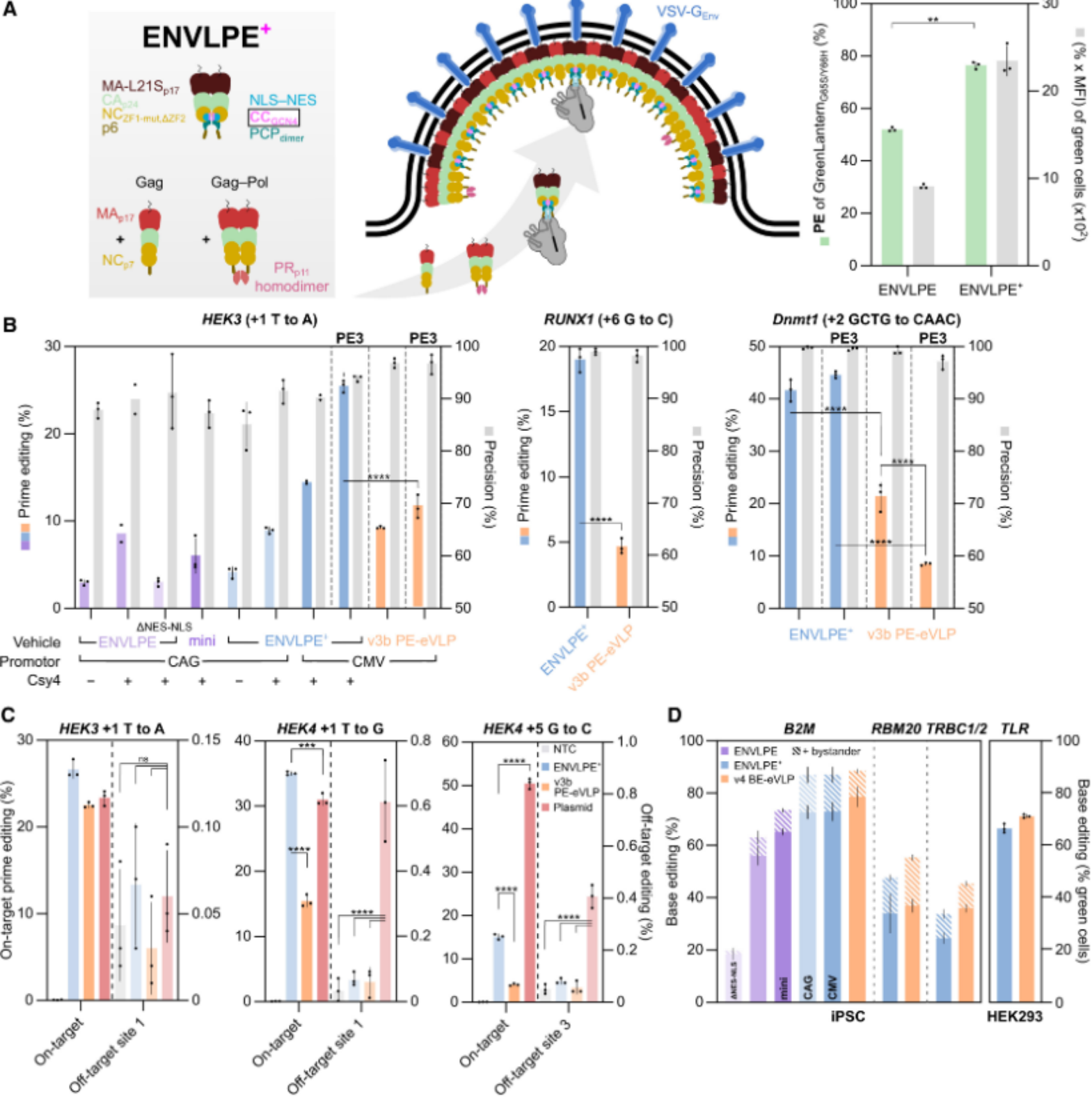
Recently, researchers from the Technical University of Munich in Germany published a research paper titled "Engineered nucleocytosolic vehicles for loading of programmable editors" in the international top academic journal Cell. The study developed a new efficient and versatile virus-like particle (VLP) delivery vector, ENVLPE, which can deliver all major RNA-guided gene editing tools (CRISPR-Cas9, base editors, prime editors) to a variety of cell types in the form of RNPs. This delivery system avoids the risk of DNA integration and demonstrates excellent editing effects in primary human T cells and two inherited retinal disease mouse models, highlighting its therapeutic potential.
One of the key challenges in the field of gene therapy is the safe and effective introduction of transgenes into cells to cure or reduce the severity of a disease. Viral vectors have proven to be one of the most effective means of achieving this goal, taking advantage of the natural ability of viruses to invade and introduce their genetic material into human cells. As a result, viral vectors are being developed for the treatment of a variety of diseases, including monogenic syndromes and cancer, and can be delivered in vivo, in situ, and ex vivo (cell therapy).
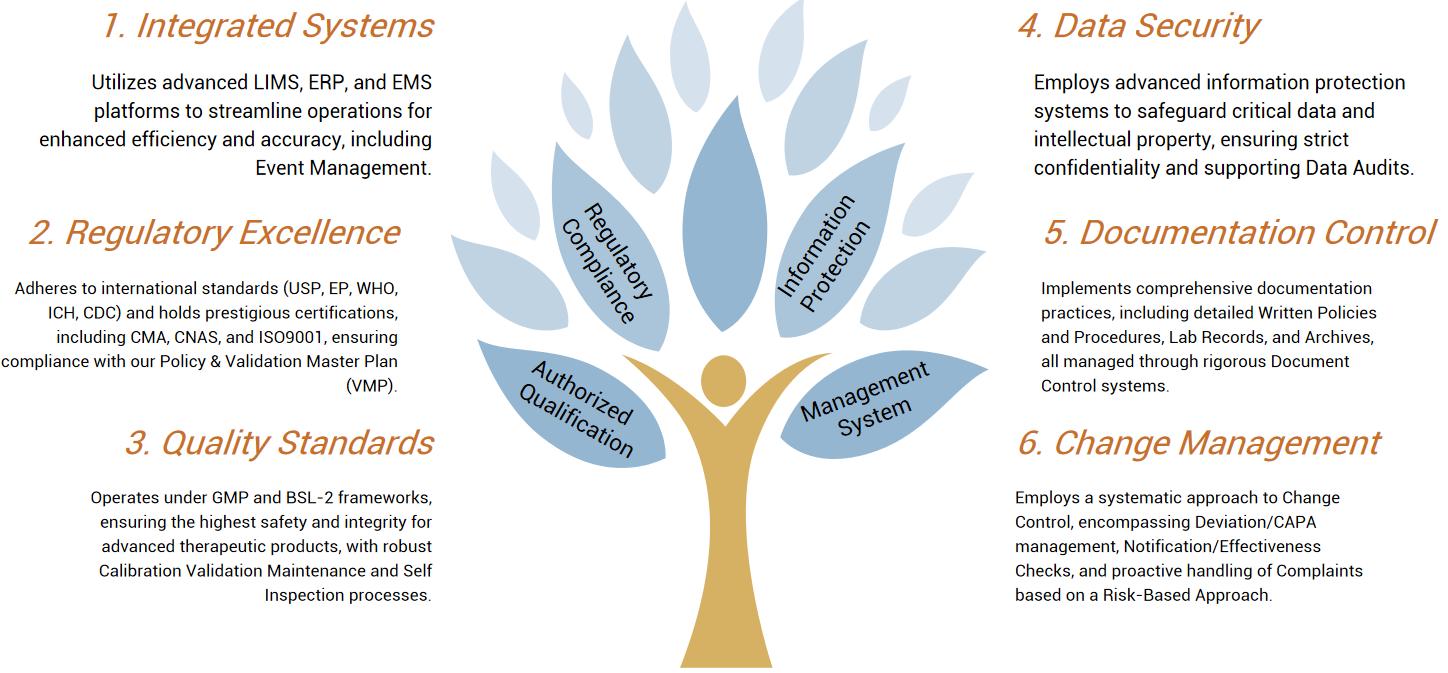
In a new study, Associate Professor Leszek Liowski and his team at the University of Sydney have identified a new method for producing a therapeutic product, chimeric antigen receptor (CAR) T cells, which has the potential to improve the treatment of cancer. The relevant research results were recently published in the journal Molecular Therapy, with the title of the paper "Tailoring capsid-directed evolution technology for improved AAV-mediated CAR-T generation".
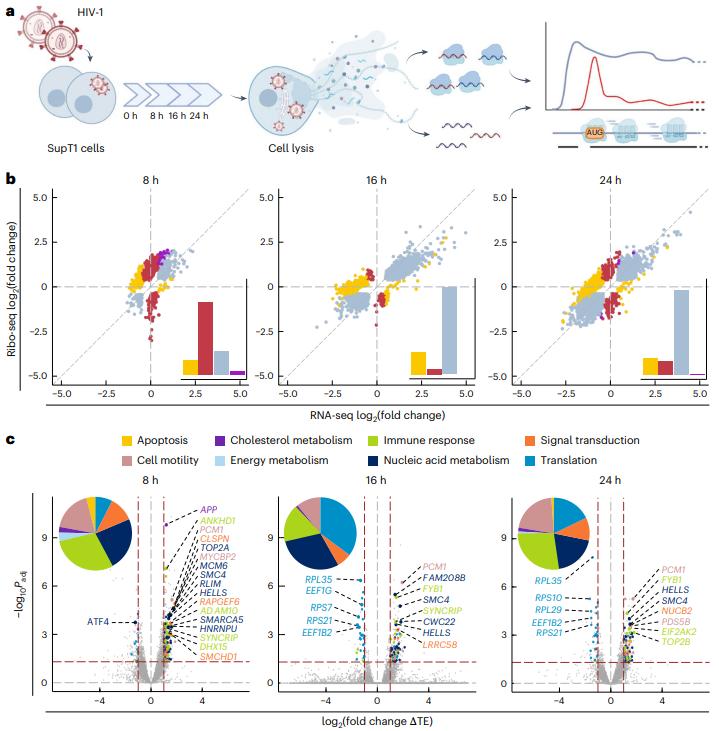
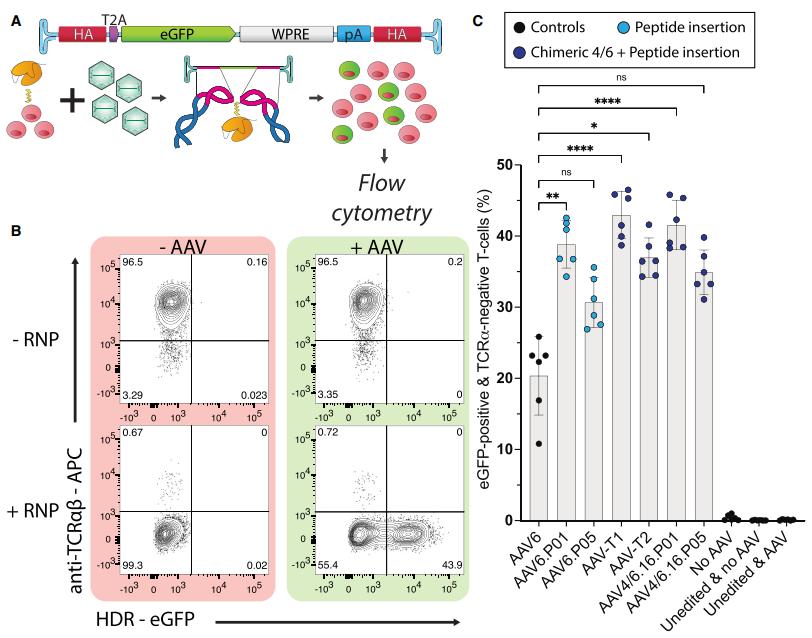
In a new study, a research team from the Helmholtz Institute for RNA Infection Research and the University of Regensburg has provided new insights into how HIV-1, the virus that causes AIDS, cleverly hijacks cellular machinery to maintain its own survival. By dissecting the molecular interactions between the virus and its host, they identified a new strategy for HIV-1 to ensure its own replication while suppressing host cell defenses. The relevant research results were published online in the journal Nature Structural & Molecular Biology in January 2025, with the title "The translational landscape of HIV-1 infected cells reveals key gene regulatory principles".
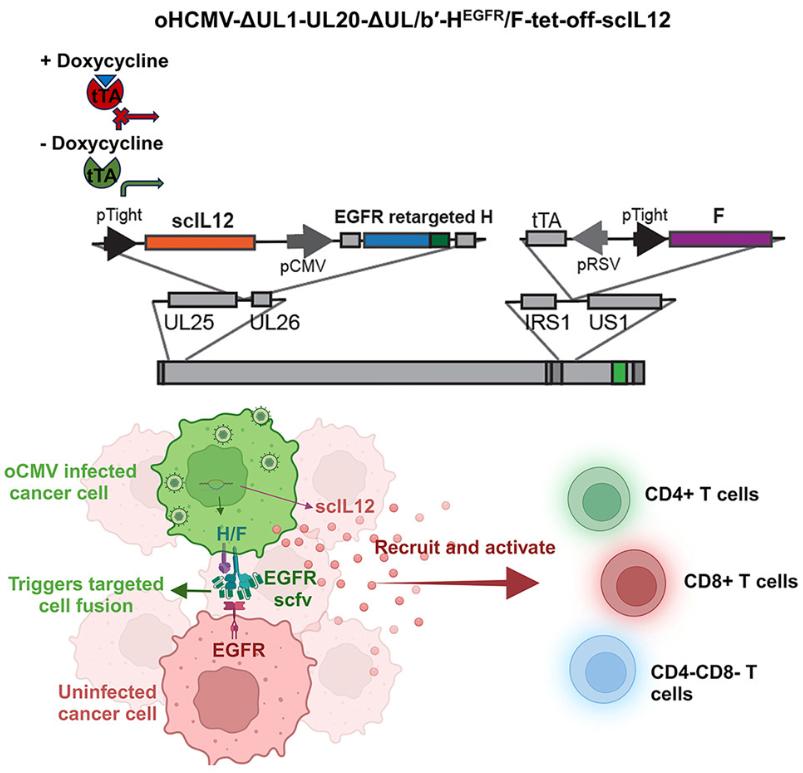
Researchers at the Mayo Clinic published a research paper titled "Oncolytic cytomegaloviruses expressing EGFR-retargeted fusogenic glycoprotein complex and drug-controllable interleukin 12" in Cell Reports Medicine. This study developed an oncolytic human cytomegalovirus expressing an EGFR retargeting fusion gene glycoprotein complex and drug-controllable IL-12, and its significant anti-tumor effect was verified in glioblastoma (GBM) models.
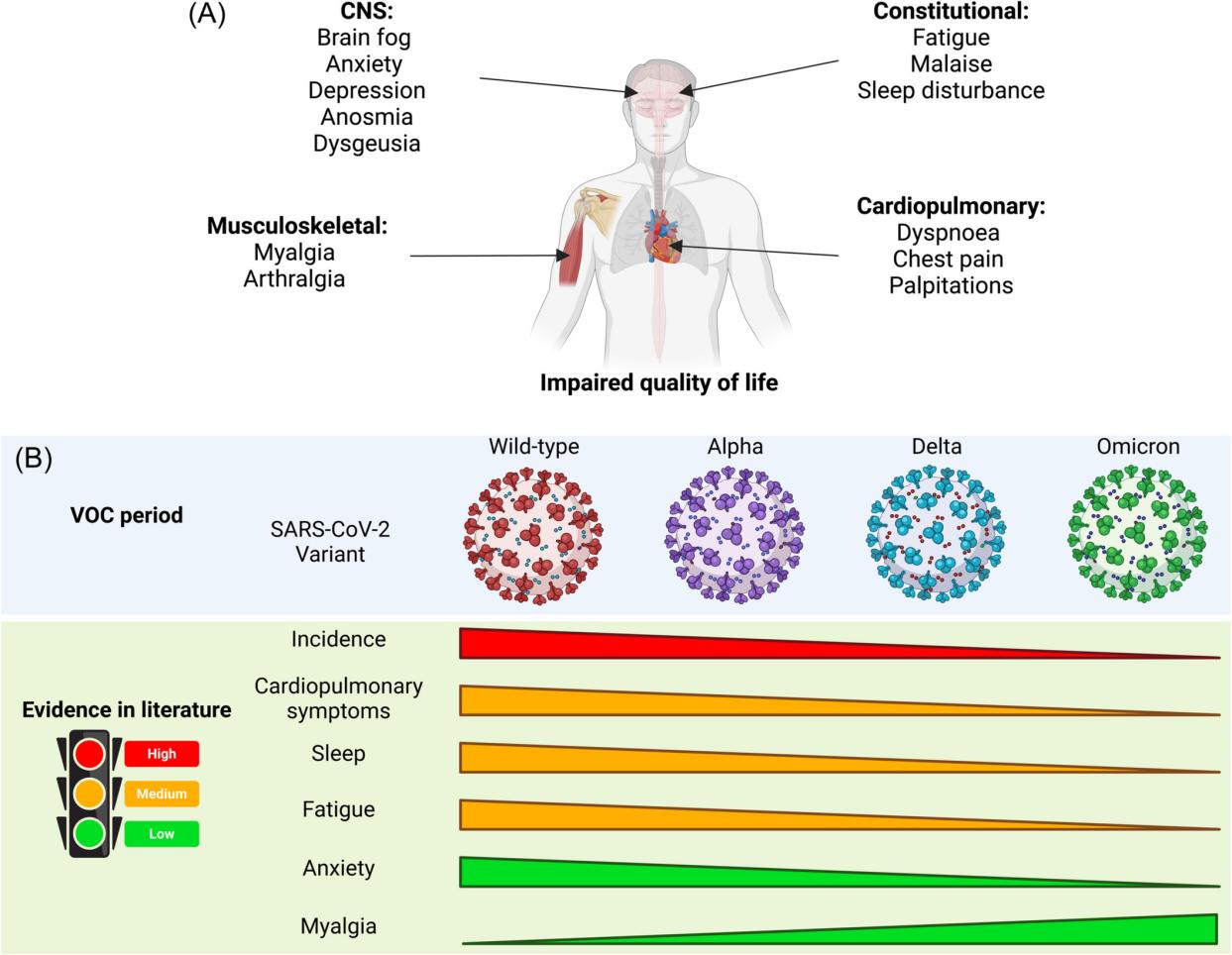
Since the outbreak of the COVID-19 pandemic in late 2019, it has had a huge impact on health, society and economy around the world. As the virus continues to spread, we realize that the impact of COVID-19 infection goes far beyond the acute phase. Some patients still suffer from persistent symptoms weeks or even months after infection, a condition known as "Long COVID" or "Post-acute sequelae of COVID-19" (PASC).
Hemophilia is an X-linked recessive inherited bleeding disorder caused by genetic defects in coagulation factor VIII or coagulation factor IX. It is divided into two main types: hemophilia A (F VIII deficiency) and hemophilia B (F IX deficiency). In a new study, researchers from Christian Medical College in Vellore, India, and other research institutions found that lentiviral vectors can be successfully used to provide gene therapy for patients with severe hemophilia A. They provide a potential alternative to adeno-associated virus (AAV)-mediated gene therapy, solving the problem of excluding patients with pre-existing anti-AAV antibodies from gene therapy. The relevant research results were published online in the New England Journal of Medicine on December 9, 2024, with the title "Lentiviral Gene Therapy with CD34+ Hematopoietic Cells for Hemophilia A".
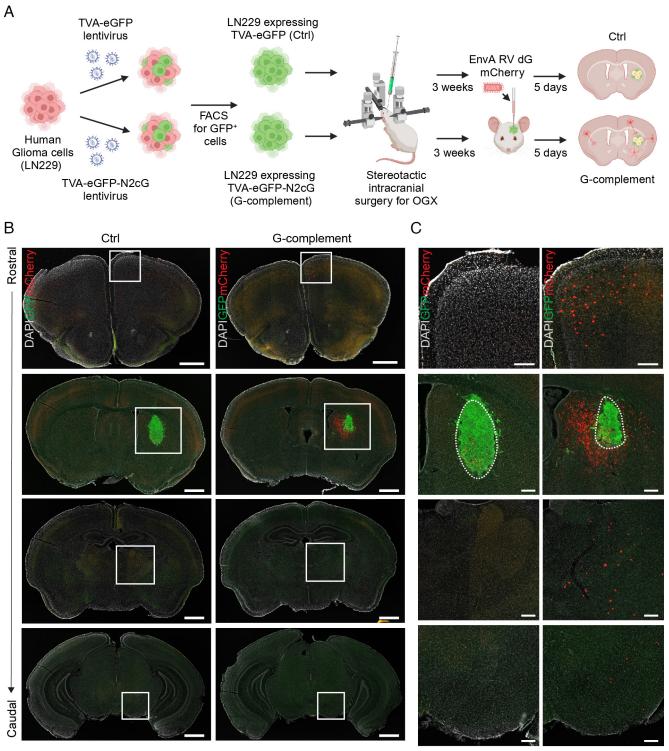
Gliomas are the most common type of brain cancer, including the deadliest form, glioblastoma. Every week, Harvard Medical School neuro-oncologist Annie Hsieh treats patients with gliomas. After Hsieh's fellow neurosurgeons remove a glioma with surgery, it often appears that no cancer cells are left. Radiation and other treatments may follow. However, gliomas often recur, not only in the original site but also in distant parts of the brain. This can harm the nervous system and, in some cases, lead to death.