Neuronal cell loss is a defining feature of Alzheimer's disease (AD), but it is currently unclear how neurons die and how this relates to other defining features of the disease. Existing in vivo AD models only partially recapitulate the neuropathology of AD, with very minimal or no neuronal cell loss.
Bart De Strooper’s team at the VIB-KU Leuven Center for Brain and Disease Research in Belgium published a research paper titled: MEG3 activates necroptosis in human neuron xenografts modeling Alzheimer’s disease in the top international academic journal Science. The study shows that in the brains of Alzheimer's disease mouse models transplanted with human or mouse neurons, only the human neurons showed severe Alzheimer's disease pathology, including neurofibrillary tangles and Necroptosis. Long non-coding RNA MEG3 is strongly up-regulated in human neurons with Alzheimer's disease lesions. Down-regulation of MEG3 through pharmacological or genetic means can rescue neuronal loss in xenografted human neurons. This discovery reveals the unique susceptibility of human neurons to Alzheimer's disease and suggests a potential therapeutic target for Alzheimer's disease.
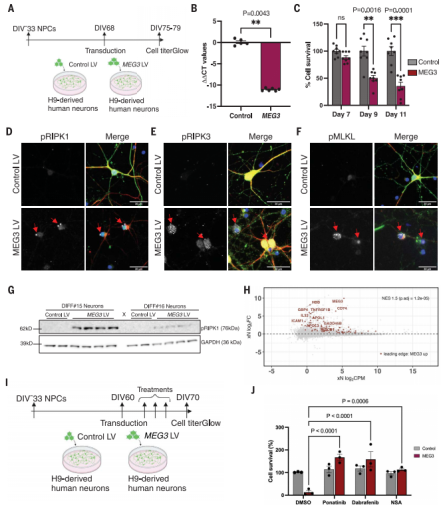
Figure 1. Long noncoding RNA MEG3 induces necroptosis in human neurons. (Balusu S, et al., 2023)
Beta-amyloid (Aβ) plaques, neurofibrillary tangles, granulovacuolar neurodegeneration (GVD), and neuronal loss are common pathological features of Alzheimer's disease. Alzheimer's disease mouse models are classic animal models for studying Alzheimer's disease, but these animal models are usually artificially induced, making it difficult to explain how these pathological characteristics are related to each other.
Indeed, fundamental questions about whether Aβ pathology can induce tau tangles and how neurons die in Alzheimer's disease remain unanswered. There are two excellent models that replicate the human pathology of Alzheimer's disease - 3D human brain organoids and xenografting human neurons in mouse brains.
In this latest study, the research team improved a previous xenograft model based on Nod-SCID mice, using the Rag2-/- (Rag2tm1.1Cgn) immunosuppressive genetic background and a single AppNL-G-F (Apptm3.1Tcs/Apptm3.1Tcs ) knock-in genes to drive Aβ pathology. The research team transplanted human stem cell-derived neural progenitor cells (NPCs) into control Rag2-/- mice, which integrated well and developed dendritic spines. Two months after transplantation, xenografted neurons already showed characteristics of some mature neuronal markers (NEUN, MAP2) and cortical markers (CTIP2, SATB2, TBR1, CUX2).
Compared with control groups transplanted with mouse neurons, mice transplanted with human neurons exhibited severe Alzheimer's disease pathology, including neurofibrillary tangles, granulovacuolar neurodegeneration (GVD), phosphorylated tau protein blood biomarkers and considerable neuronal loss. This suggests that there are unknown human-specific features that define the susceptibility of human neurons to Aβ pathology.
Transcriptomic analysis revealed that neuron-specific maternally expressed gene 3 (MEG3), a long non-coding RNA (lncRNA), was strongly upregulated approximately 10-fold in diseased human neurons. It is worth mentioning that this neuron-specific long non-coding RNA is also upregulated 2-3 times in Alzheimer's disease patients.
Further studies have shown that overexpression of MEG3 in vitro is sufficient to induce necroptosis in human neurons. Down-regulation of MEG3 and inhibition of necroptosis using pharmacological or genetic manipulation of receptor-interacting protein kinase 1 (RIPK1), RIPK3, or mixed lineage kinase domain-like protein (MLKL) rescued neuronal cell loss in xenografted human neurons. This model proposes potential treatments for AD and reveals human-specific susceptibility to AD.
Taken together, this latest study published in Science demonstrates that Aβ pathology can induce necroptosis in human neurons, and that acute upregulation of MEG3 is a key factor. Therefore, neuronal loss in Alzheimer's disease can be rescued by downregulating the expression of MEG3 or inhibiting the necroptosis pathway, which also brings new potential targets and methods for the treatment of Alzheimer's disease.
Reference
Balusu S, et al. MEG3 activates necroptosis in human neuron xenografts modeling Alzheimer’s disease. Science, 2023, 381(6663): 1176-1182.