In the field of modern biotechnology, the CRISPR/Cas system has become one of the important tools for gene editing. Especially for RNA editing, the CRISPR/Cas13 system shows great potential. Among the Cas13 family, Cas13d is considered to be the most active isoform in mammalian cells. However, Cas13d is insufficiently active in the cytosol of mammalian cells, which limits its efficiency in applications such as programmed antiviral therapy. Since most RNA viruses only replicate in the cytoplasm, the effectiveness of existing Cas13d-based antiviral methods is limited by uncontrolled leakage of nucleic acids.
In order to solve this limitation, the recent study "Engineered, nucleocytoplasmic shuttling Cas13d enables highly efficient cytosolic RNA targeting" published in Cell Discovery designed and developed a nucleocytoplasmic shuttling Cas13d (Cas13d-NCS), which can transfer CRISPR RNA (crRNA) in the nucleus to the cytoplasm to achieve efficient targeting of cytoplasmic RNA.
| CATALOG NO. | PRODUCT NAME | INQUIRY |
|---|---|---|
| CCR-001 | Cas9 mRNA | Inquiry |
| CCR-002 | Cas9 D10A mRNA | Inquiry |
| CCR-003 | SpCas9-GFP mRNA | Inquiry |
| CCR-004 | SaCas9 mRNA | Inquiry |
| CCR-005 | LwaCas13a mRNA | Inquiry |
| CCR-006 | Cas13d mRNA | Inquiry |
By screening various designed shuttling proteins and evaluating multiple design parameters, the research team found that Cas13d-NCS performed well in degrading messenger RNA (mRNA) and self-replicating RNA from Venezuelan equine encephalitis (VEE) RNA virus. Further experiments confirmed that Cas13d-NCS can completely block the replication of different SARS-CoV-2 strains. Therefore, Cas13d-NCS not only provides a new strategy for the CRISPR system to precisely control subcellular localization, but also provides the possibility to develop new programmed therapies against cytoplasmic RNA viruses.
The success of this study lies in the precise molecular modification of Cas13d. By adding a nuclear localization sequence (NLS) and a nuclear export sequence (NES) to its structure, it can be freely transferred between the nucleus and the cytoplasm. In addition, by systematically studying different NLS/NES ratios, the research team optimized the design of Cas13d-NCS. This allows Cas13d-NCS to ensure sufficient nuclear import to bind crRNA while also effectively exporting it into the cytoplasm to improve the efficiency of targeting mRNA that is mainly located in the cytoplasm.
In conclusion, the development of Cas13d-NCS not only provides a new dimension for the application of CRISPR technology, but also demonstrates the prospect of improving therapeutic efficacy through precise control of protein subcellular localization. In addition, the application potential of Cas13d-NCS goes far beyond antiviral therapy and may also include the treatment of various RNA-related diseases. This marks an important step toward more refined and customized molecular biological tools and therapeutic strategies.
Highlights
- Development of nucleocytoplasmic transport Cas13d: The Cas13d-NCS developed in the study can effectively transfer CRISPR RNA (crRNA) from the nucleus to the cytoplasm, achieving targeted degradation of RNA in the cytoplasm.
- Improved antiviral efficacy against cytoplasmic RNA viruses: This system shows significant antiviral activity against VEE viruses and multiple strains of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), and completely block the replication of these viruses.
- Potential clinical application prospects: Cas13d-NCS has demonstrated its great potential in accurately regulating RNA targeting at the subcellular level and developing new molecular tools and treating RNA-related diseases.
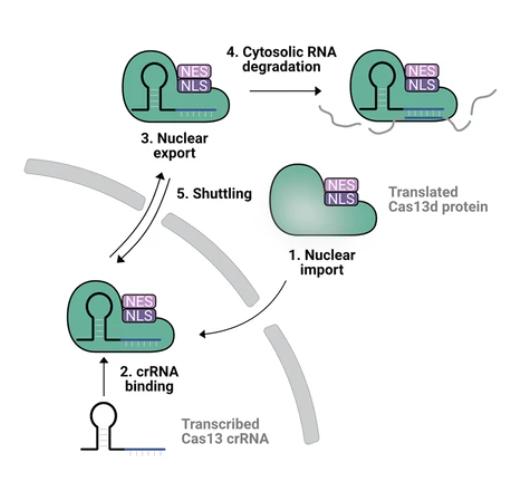
Figure 1. Schematic illustration of nucleocytoplasmic shuttling Cas13d to transport crRNAs to the cytosol. (Gruber C, et al. 2024)
Strategies
In terms of specific methods, the first step is to genetically engineer the Cas13d protein and add a nuclear localization signal sequence (Nuclear Localization Sequence, NLS) and a nuclear export signal sequence (Nuclear Export Sequence, NES). Through the configuration of these sequences, the improved Cas13d can form a complex with crRNA in the nucleus, and then transport it out of the nucleus through the NES to achieve activity in the cytoplasm. The research team screened through various designed versions of the protein mix and further studied the design parameters for the best performance of the system. They found that the efficiency of Cas13d-NCS in degrading mRNA and self-replicating RNA derived from VEE viruses in the cytoplasm was much higher than that of traditional nuclear-localized Cas13d.
In addition, the effectiveness of different Cas13d variants in targeting RNA viruses was compared by flow cytometry, and the results showed that Cas13d-NCS can significantly improve the targeting and degradation efficiency of cytoplasmic RNA. Ultimately, this new Cas13d was used to block the replication of different SARS-CoV-2 strains, showing its great potential in developing targeted therapies for RNA viruses. Overall, the development of Cas13d-NCS not only optimizes the subcellular localization of Cas13d, but also greatly improves its application potential in RNA virus prevention and treatment, providing a new strategy for precise cellular RNA targeting using CRISPR technology.
Limitation
Despite its promise as a RNA-targeting technology, Cas13d has very limited activity in the cytoplasm and is mainly confined to the nucleus. This limitation significantly affects its use in programmed antiviral therapies, since most RNA viruses replicate in the cytoplasm. This means that existing Cas13d-based antiviral strategies mainly rely on uncontrolled leakage of nucleic acids, thus limiting their effectiveness. Furthermore, the nuclear localization of Cas13d guide RNA (crRNA) is the fundamental reason for the preferential nuclear activity of Cas13d. Since mRNA is rapidly transported to the cytoplasm after transcription, the time window for the traditional Cas13d-NLS system to recognize and bind target mRNA is very short.
Reference
Gruber C, et al. Engineered, nucleocytoplasmic shuttling Cas13d enables highly efficient cytosolic RNA targeting. Cell Discovery, 2024, 10(1): 42.

