Recently, a research team from China published a research paper in the journal Science Advances titled: Cryo-shocked tumor cells deliver CRISPR-Cas9 for lung cancer regression by synthetic lethality. This study developed a cell delivery vector that efficiently delivers the CRISPR-Cas9 gene editing system to the lungs.
This study used rapid liquid nitrogen treatment of lung cancer cells to eliminate the pathogenicity of cancer cells while retaining their cellular structure and surface receptor activity. Using these "cryo-shocked" lung cancer cells as delivery vehicles, the CRISPR-Cas9 system was efficiently delivered to lung cells through passive targeting and active targeting, effectively reducing the expression level of cyclin-dependent kinase 4 gene (CDK4). Inducing synthetic lethality in KRAS-mutated non-small cell lung cancer (NSCLC) resulted in tumor elimination and prolonged survival in mouse models.
| Cat.No. | Product Name | Price |
|---|---|---|
| CSC-DC002874 | Panoply™ Human CDK4 Knockdown Stable Cell Line | Inquiry |
| CSC-RT1960 | CDK4 Knockout Cell Line-HeLa | Inquiry |
| CSC-RT2742 | Human CDK4 Knockout Cell Line-HCT116 | Inquiry |
| CSC-SC002874 | Panoply™ Human CDK4 Over-expressing Stable Cell Line | Inquiry |
| AD03471Z | Human CDK4 adenoviral particles | Inquiry |
| LVIM011Z | Human CDK4 Lentivirus (CMV, Puro) | Inquiry |
| LVIM012Z | Human CDK4 Lentivirus (CMV) | Inquiry |
| LVIM023Z | EF1a-hCDK4(GFP, Puro) Lentiviral Particles | Inquiry |
| LVIM024Z | EF1a-hCDK4(RFP, Bla) Lentiviral Particles | Inquiry |
| CLKO-1819 | CDK4 KO Cell Lysate-HeLa | Inquiry |
| CLOE-1223 | Human CDK4 Insect Cell Lysate | Inquiry |
This study developed a passive and active dual-targeted lung-targeted CRISPR-Cas9 drug delivery strategy to induce synthetic lethality in cancer cells of non-small cell lung cancer (NSCLC) by knocking down CDK4 in tumors. Synthetic lethality is defined as the simultaneous inactivation of two genes resulting in cell death, whereas the loss of function of a single gene has little effect on cell survival. Often, tumor cells carry oncogene mutations, which make them more dependent on certain genes to maintain cellular homeostasis. In theory, these tumor cells carrying a specific gene mutation could be selectively killed by a drug that inhibits the synthetic lethal interaction of another gene, while normal cells are protected from the effects of the drug due to the lack of the specific gene mutation.
Therefore, synthetic lethality offers a promising therapeutic strategy to target some undruggable oncogenes while mitigating damage to normal tissues and cells. There are already some synthetic lethal interactions used in clinical trials and treatments. KRAS is one of the most commonly mutated genes in many types of cancer, including NSCLC. Some recent studies have discovered multiple synthetic lethal interactions in KRAS mutant NSCLC, providing an alternative approach to enhance the clinical therapeutic effect of NSCLC.
A549 cells are a typical KRAS mutant NSCLC cell. After rapid freezing and inactivation by liquid nitrogen treatment and elimination of pathogenicity, A549 cells were used as in vivo CRISPR-Cas9 delivery vectors. Due to its intact cellular structure and retained cell surface glycoprotein CD44, this cell carrier can achieve active targeted delivery to lung cells via passive capture in pulmonary capillaries as well as CD44-mediated cell interactions and adhesion.
The research team co-assembled the pCas9/gCDK4 plasmid targeting CDK4 knockout with lipofectamine transfection reagent to form a nanoformulation, and anchored it to the surface of A549 cells treated with liquid nitrogen through electrostatic interaction. The NSCLC tumor-bearing mouse model was then injected through the tail vein. Compared with direct systemic injection of CRISPR-Cas9 nanoparticles, the delivery efficiency of this new delivery vector increases by nearly 4 times. The expression level of CDK4 in tumors decreased significantly without affecting normal cells. CDK4 knockdown induces synthetic lethality in KRAS mutant NSCLC and extends survival in mouse models.
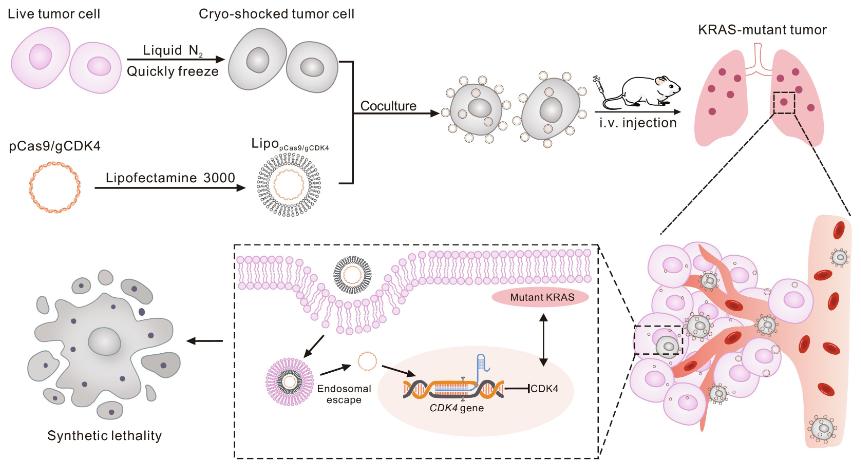
Figure 1. Schedule of LNT cells delivery of CRISPR-Cas9 nanoparticles for KRAS-mutant NSCLC treatment. (Liu F, et al. 2024)
Another advantage of the technology is that cells from tumor tissue harvested from surgical resection, needle biopsy or other means can be reused and can be frozen and used as delivery vehicles for gene-editing tools to treat cancer. In addition, tumor cells treated with rapid liquid nitrogen retain tumor antigens on the cell surface, so in addition to serving as delivery vectors, they may also serve as vaccines for tumor immunotherapy.
References
Liu F, et al. Cryo-shocked tumor cells deliver CRISPR-Cas9 for lung cancer regression by synthetic lethality. Science Advances, 2024, 10(13): eadk8264.
Ci T, et al. Cryo-shocked cancer cells for targeted drug delivery and vaccination. Science advances, 2020, 6(50): eabc3013.
Shi J, et al. Lyophilized lymph nodes for improved delivery of chimeric antigen receptor T cells. Nature Materials, 2024: 1-10.

