Long noncoding RNAs (lncRNAs) contain more than 200 nucleotides, lack protein-coding potential, and are widely transcribed in eukaryotic cell genes. At present, studies have shown that lncRNAs play a key role in gene expression in various cells and biological processes. Unlike conserved mRNAs, lncRNAs lack high sequence or secondary structure conservation. lncRNA conservation can also occur at the position and mechanism-of-action levels. Transcription of positionally conserved lncRNAs with nearby conserved coding genes among different species has been recognized as an indicator of potential functional significance. However, whether lncRNA processing is conserved and how processing contributes to its compartmentalization and function in different species remain unexplored.
A few days ago, in a research report published in the international journal Cell, the research team of Ling-Ling Chen from Shanghai Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences found that different processing methods of lncRNAs homologous sequences will trigger different subcellular localization in human and mouse embryonic stem cells, which will subsequently lead to their functional differences in the regulation of pluripotency in different species. Related research results indicate that conserved lncRNAs may realize functional evolution through non-conservative RNA processing and localization processes. 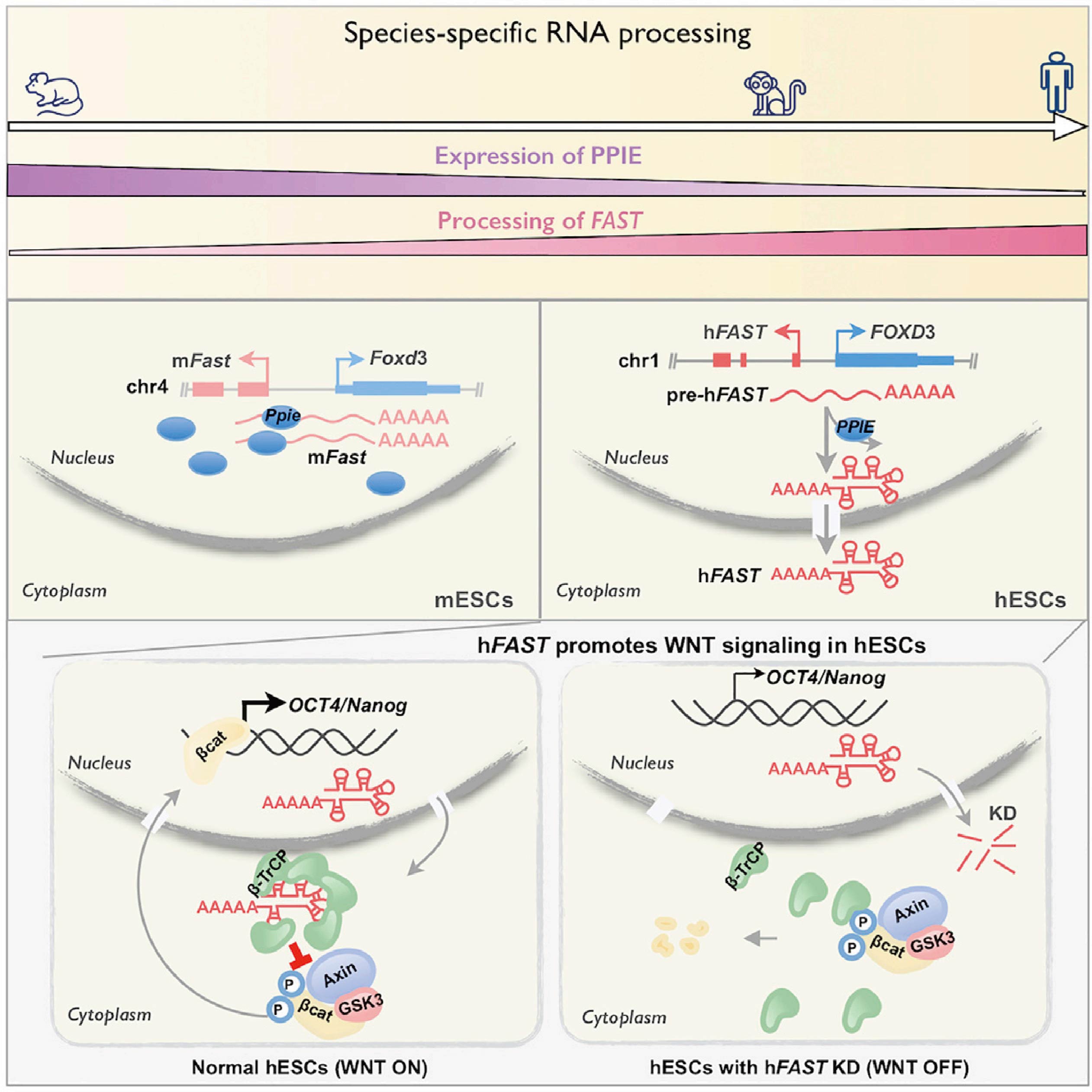
(Chun-Jie Guo, et al., Cell, 2020).
By analyzing RNAs isolated from the cytoplasm and nucleus of human and mouse embryonic stem cells (ESCs), the researchers revealed different subcellular localization patterns of sequence-conserved and position-conserved lncRNAs in these cells. And compared with mice, a higher percentage of human embryonic stem cells (hESCs) in lncRNAs will be spliced and exported to the cytoplasm, which is very important for the pluripotency of hESCs.
FOXD3 antisense transcript (FAST, FOXD3 antisense transcript 1) is a conserved lncRNAs, but is not conserved in its processing and localization. In hESCs, the cytoplasmic-localized hFAST can bind to the WD40 repeat domain on the E3 ubiquitin ligase β-TrCP, thereby inhibiting the degradation of β-catenin, leading to activated WNT signaling, required for pluripotency. However, mFAST has nuclear retention properties in mouse embryonic stem cells (mESCs), which is necessary for self-renewal.
Subsequently, the researchers further found that the key processing factor peptidylprolyl isomerase E (PPIE) may be mainly responsible for different processing methods of lncRNAs in various species. PPIE will be highly expressed in mESCs, while it will inhibit the processing and nuclear export of various lncRNAs (including mFAST). On the contrary, the low expression of PPIE in hESCs and mESCs will promote the efficient splicing of hFAST and export into the cytoplasm, and hFAST can promote the self-renewal of stem cells. These findings reveal that lncRNA processing and localization are previously under-appreciated contributors to the rapid evolution of function.
Reference
- Chun-Jie Guo, et al. Distinct Processing of lncRNAs Contributes to Non-conserved Functions in Stem Cells, Cell (2020).

