Recently, researchers at Johns Hopkins University in the United States discovered that lymphocyte activation gene-3 (Lag-3) in neurons is a specific receptor for tau PFF in the brain and can promote the spread of tau PFF between neurons. In addition, they observed that Lag-3 knockout in neurons significantly reduced the endocytosis of tau PFF and reduced its spread between neurons. Researchers also observed a similar phenomenon in conditional knockout mice of Lag-3 neurons. This result also means that Lag-3 may be used as a potential therapeutic target for AD and related tauopathies. Relevant research was published in Advanced Science.
View all Immune Checkpoint Stable Cell Lines
In fact, the research team discovered in 2016 that Lag-3, a cell surface receptor, can specifically bind to the PFF of α-synuclein, and inhibiting Lag-3 can inhibit α-synapsis. Uptake of nuclear proteins and reduce pathological aggregation of α-synuclein. On this basis, the researchers wanted to know whether Lag-3 could also specifically bind to tau PFF.
| Cat.No. | Product Name | Price |
|---|---|---|
| CSC-DC008515 | Panoply™ Human LAG3 Knockdown Stable Cell Line | Inquiry |
| CSC-RO0009 | Human LAG3 Stable Cell Line-CHO | Inquiry |
| CSC-RO0107 | Human LAG3 Stable Cell Line-HEK293T | Inquiry |
| CSC-RO0246 | Monkey LAG3 Stable Cell Line-HEK293T | Inquiry |
| CSC-RO0941 | Human LAG3 Stable Cell Line - 3A9 | Inquiry |
| CSC-RO1020 | Monkey LAG3 Stable Cell Line-HEK293 | Inquiry |
| CSC-RT1454 | Human LAG3 Knockout Cell Line-HeLa | Inquiry |
| CSC-SC008515 | Panoply™ Human LAG3 Over-expressing Stable Cell Line | Inquiry |
| CSC-SC008515-1 | Human LAG3 Stable Cell Line - HEK293 | Inquiry |
| AD08987Z | Human LAG3 adenoviral particles | Inquiry |
| LV16717L | human LAG3 (NM_002286) lentivirus particles | Inquiry |
| CLKO-1304 | LAG3 KO Cell Lysate-HeLa | Inquiry |
| CLOE-1939 | Rat Lag3 HEK293 Cell Lysate | Inquiry |
Therefore, in this study, the researchers first extracted AD-tau from the brains of AD patients, and then used ultrasound processing to obtain tau PFF. The researchers then labeled tau with biotin to distinguish endogenous tau. In subsequent cell surface binding assays, the researchers found that Lag-3 could bind to biotin-labeled tau PFF (dissociation constant K = 158 nm), but not to the biotin-labeled tau monomeric form.
Further, the researchers compared the KD difference between normal neurons of wild-type mice and neurons of wild-type mice lacking Lag-3 with biotin-labeled tau PFF. They concluded that Lag-3 in neurons can specifically bind to biotin-labeled tau PFF. And biotin-labeled tau PFF preferentially binds to the D1 domain of the extracellular domain of Lag-3.
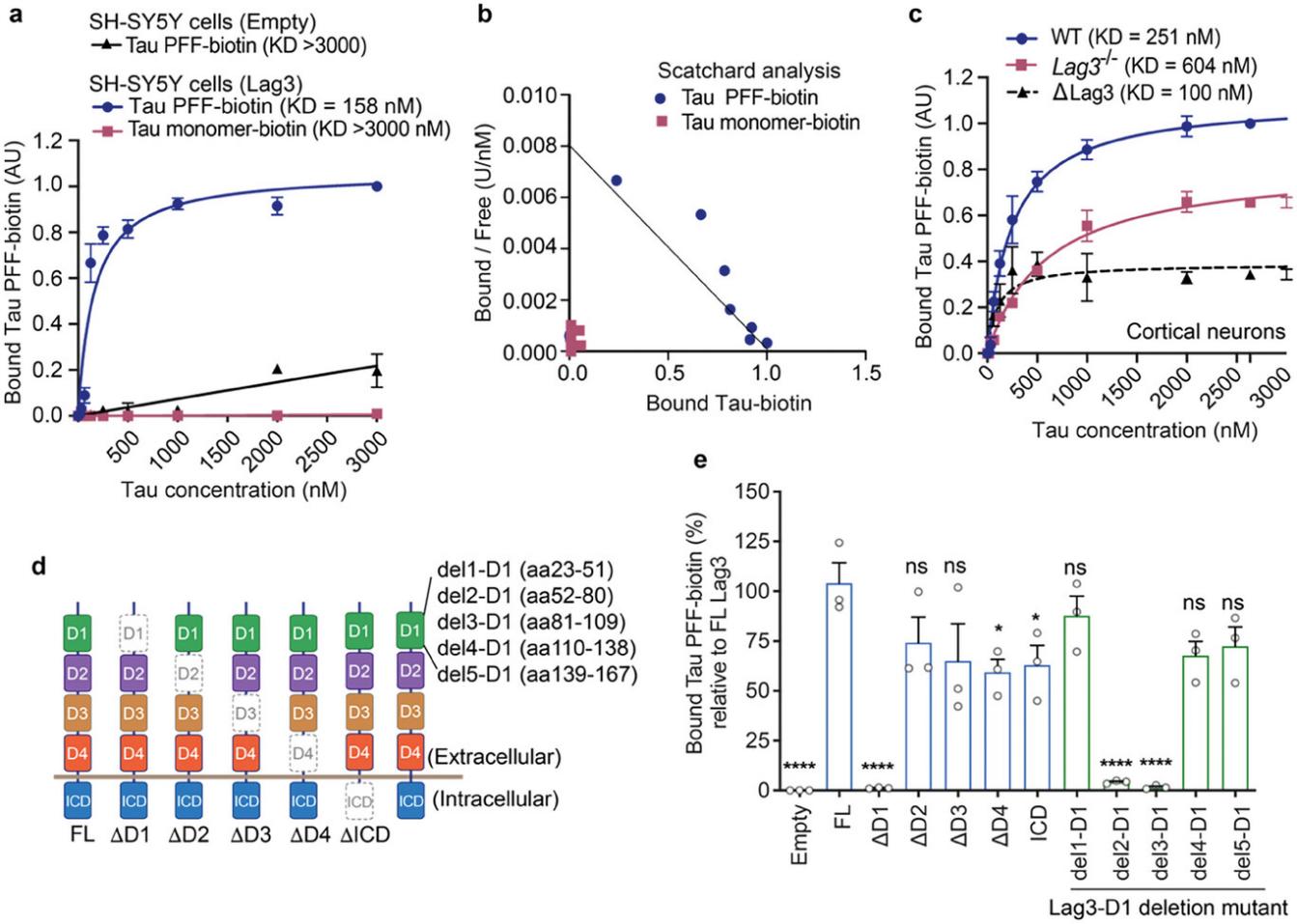
Figure 1. Lag3 binds with Tau PFF, but not Tau monomer. (Chen C, et al. 2024)
Next, the researchers wanted to know whether Lag-3 was involved in the endocytosis of tau PFF. After combining tau PFF with pHrodo red dye, the researchers found that tau-pHrodo PFF fluoresced in wild-type mouse cortical neurons, while neurons lacking Lag-3 had little fluorescence signal. In contrast, the fluorescence signal of neurons overexpressing Lag3 was enhanced. This shows that Lag-3 is indeed involved in the endocytosis of Tau PFF, and Lag-3 gene knockout can significantly inhibit its endocytosis.
Tau PFF will spread in neurons. In order to observe the role of Lag-3 in spreading between tau PFF neurons, the researchers used a microfluidic device (three chambers in series) to simulate the spread between neurons. It was found that Lag-3 plays a key role in the interneuronal spread of tau PFF, i.e., in neuronal devices lacking Lag-3, the amount of insoluble tau protein in the last compartment was reduced. In contrast, insoluble tau protein content was significantly increased in neuronal devices overexpressing Lag-3.
Finally, the researchers also verified the relationship between Lag-3 and tau PFF in vivo. By constructing neuron-specific Lag-3 knockout mice and injecting tau PFF into the hippocampus of these mice, the researchers evaluated the expression levels of phosphorylated tau protein in the ventral hippocampus, entorhinal cortex, and amygdala of these mice. The results found that 9 months after injection, the expression levels of phosphorylated tau protein in the above three regions were significantly lower compared with the control group. In multiple behavioral tests (mainly the anxiety-related behavioral test and the three-chamber social interaction test to assess cognitive function), the behavioral deficits of neuron-specific Lag-3 knockout mice were also significantly improved.
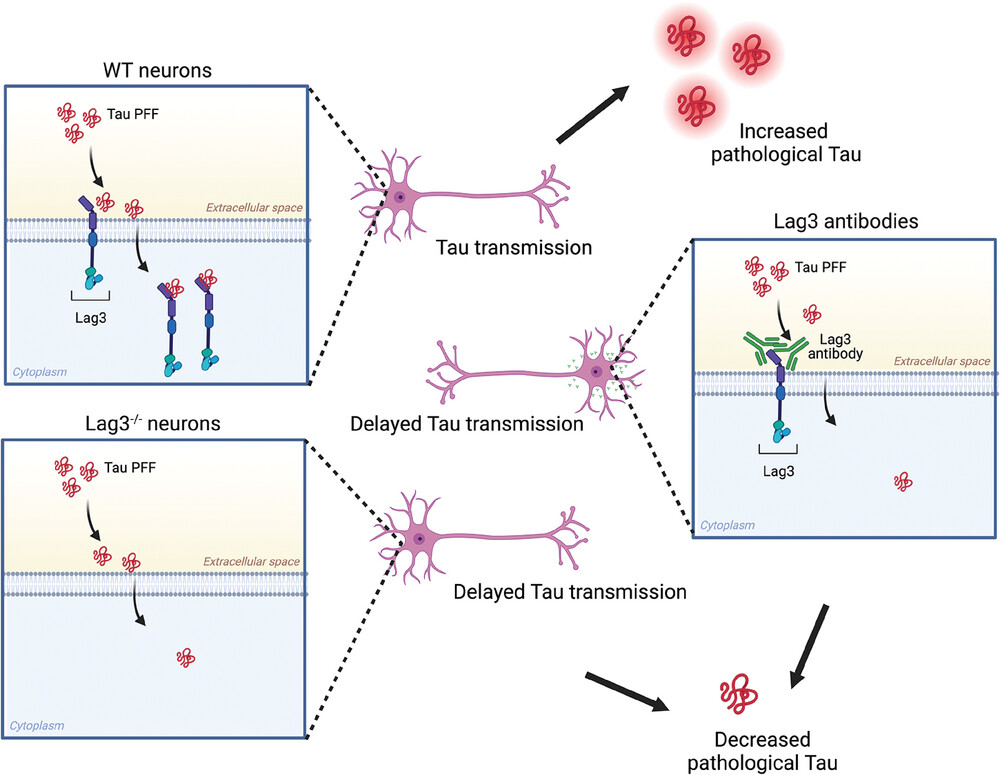
Figure 2. Proposed Model. (Chen C, et al. 2024)
In conclusion, this study found that Lag-3 can specifically bind to tau PFF and promote the transmission of pathological tau protein between neurons. Inhibiting Lag-3 can prevent this phenomenon. Given that LAG-3 has also been a target of much attention in tumor immunotherapy in recent years, the author of this article stated that anti-LAG-3 antibodies may be a very promising method for the treatment of AD and tau disease in the future.
Reference
Chen C, et al. Lymphocyte‐Activation Gene 3 Facilitates Pathological Tau Neuron‐to‐Neuron Transmission. Advanced Science, 2024: 2303775.

