Toll-like receptor 4 (TLR4) is a central regulator of the body's innate immunity. It can mainly recognize bacterial lipopolysaccharide cell wall components and induce the release of cytokines. Recently, in a research report titled "RHBDL4-triggered downregulation of COPII adapter protein TMED7 suppresses TLR4-mediated inflammatory signaling" published in Nature Communications, scientists from the University of Cologne and other institutions found that the intramembrane protease RHBDL4 may be able to effectively prevent the body's excessive immune response. In the article, the researchers found that cleavage of cargo receptors by so-called intramembrane proteases reduces the localization of central immune receptors on the cell surface, thereby reducing the risk of an overreaction by the body's immune system.
Intramembrane proteases are active proteins located in the cell membrane. They form a special group of proteases because they can cleave proteins within the cell membrane. Researchers have not yet fully described the unusual protease involved, and only a few molecules are known to be capable of cleavage, so their function is unknown. One such intramembrane protease is RHBDL4, which is located in the endoplasmic reticulum. The endoplasmic reticulum is a large membrane system in the cell that is primarily responsible for the correct folding of newly synthesized proteins and their subsequent delivery into the secretory pathway. While searching for the substrate and molecular function of RHBDL4, the researchers discovered a mechanism that regulates the body's innate immune system, which involves cleaving transporters to block the trafficking of immune receptors to the cell surface.
To gain an initial understanding of RHBDL4's physiological function, the researchers used mass spectrometry to analyze the substrate in tissue culture cells. Among the candidate substrates are a variety of so-called cargo receptors, which are required for the transport of specific proteins in the secretory pathway. Contrary to long-held assumptions, most proteins are not simply secreted from the endoplasmic reticulum in a nonspecific manner but must be selected and transported by cargo receptors. One such cargo receptor, TMED7, has been characterized as a substrate of RHBDL4. By cleaving this cargo receptor, cells can precisely and rapidly control the transport of specific proteins, allowing the cell to adapt to given environmental conditions. The molecular mechanisms behind the regulation of transport by cleaving cargo receptors by intramembrane proteases have not yet been described.
This finding is interesting because the cargo receptor TMED7 transports a central immune receptor (TLR4) in the body's innate immune system to the cell surface. When TLR4 comes into contact with components of the bacterial cell wall, it induces an immune response (a defense response). Cleavage of the cargo receptor TMED7 by RHBDL4 results in a small number of TLR4 molecules reaching the cell surface, thereby reducing the immune response to bacterial cell wall components.
Since excessive activation of TLR4 can damage the organism and even promote the occurrence of blood poisoning (sepsis), the immune response mediated by TLR4 must be strictly regulated by the body. After conducting studies using mouse models, the researchers found that RHBDL4 is very important in preventing excessive activation of TLR4, which may protect the body. When the receptors are stimulated, the cells produce more RHBDL4. This is why there is a negative feedback loop, as it prevents excessive immune responses from receptors from damaging the body.
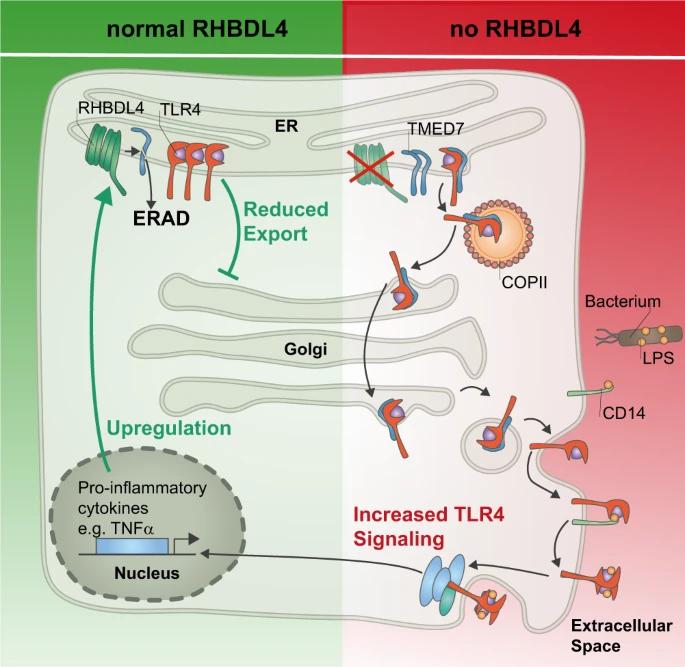
Figure 1. Model of RHBDL4-mediated negative feedback regulation of TLR4 signaling. (Knopf J D, et al. 2024)
To learn more about the clinical relevance of RHBDL4's regulation of transport, the researchers conducted a joint study and found that this regulation is particularly important when exposed to bacteria such as Mycobacterium tuberculosis. In addition, researchers have linked genetic mutations in the protease RHBDL4 to Kawasaki syndrome, an immunological disorder associated with an overreaction of the body's immune system that often affects children. This research program demonstrates the use of very simple models such as tissue culture cells to reveal new insights into human biology and disease.
Reference
Knopf J D, et al. RHBDL4-triggered downregulation of COPII adaptor protein TMED7 suppresses TLR4-mediated inflammatory signaling. Nature Communications, 2024, 15(1): 1528.

