Recently, researchers from the Technical University of Munich in Germany published a research paper titled "Engineered nucleocytosolic vehicles for loading of programmable editors" in the international top academic journal Cell. The study developed a new efficient and versatile virus-like particle (VLP) delivery vector, ENVLPE, which can deliver all major RNA-guided gene editing tools (CRISPR-Cas9, base editors, prime editors) to a variety of cell types in the form of RNPs. This delivery system avoids the risk of DNA integration and demonstrates excellent editing effects in primary human T cells and two inherited retinal disease mouse models, highlighting its therapeutic potential.
Although CRISPR-Cas9 gene editing technology, as well as the new generation of base editors and prime editors, are mature, traditional delivery methods have obvious shortcomings when applied to disease treatment:
(1) Commonly used lentiviral vectors or adeno-associated virus (AAV) vectors have integration risks and may also trigger immune responses. In addition, the vector capacity is limited and it is difficult to load base editors and prime editors.
(2) Lipid nanoparticles (LNPs) will accumulate in the liver, and are usually difficult to target specific organs or cells. The delivery efficiency is insufficient, resulting in unstable gene editing efficiency.
(3) There is a risk of long-term expression: the gene editing tools delivered (especially those delivered by viral vectors) exist in the body for a long time, which may lead to off-target effects.
In this latest study, the research team chose virus-like particles (VLPs), which are a type of "empty shell" without viral genetic material. They can simulate the infection ability of the virus while avoiding potential safety hazards.
Virus-like particle (VLP) delivery system has several advantages. It can use the diversity of known viruses with different tropisms to adjust its targeting to cells or organs/tissues. VLP can also encapsulate and deliver gene editors in the form of ribonucleoprotein (RNP), which does not contain DNA components, so there is no risk of insertion mutations. In addition, the host immune response will not be activated by the continuous expression of viral components or transgenic components.
| Cat.No. | Product Name | Price |
|---|---|---|
| VLP-AAV009 | AAV-DJ Virus-Like Particles (Empty Capsids) | Inquiry |
| VLP-AAV010 | AAV-DJ/8 Virus-Like Particles (Empty Capsids) | Inquiry |
| VLP-N-00001 | Human CCR1 Virus-Like Particles | Inquiry |
| VLP-N-00002 | Human CCR2 Virus-Like Particles | Inquiry |
| VLP-N-00003 | Human CCR5 Virus-Like Particles | Inquiry |
| VLP-N-00004 | Human CCR6 Virus-Like Particles | Inquiry |
| VLP-N-00005 | Human CXCR3 Virus-Like Particles | Inquiry |
| VLP-N-00006 | Human CXCR4 Virus-Like Particles | Inquiry |
| VLP-N-00007 | Mouse CXCR4 Virus-Like Particles | Inquiry |
| VLP-N-00008 | Human GLP1R Virus-Like Particles | Inquiry |
| VLP-N-00009 | Human HVCN1 Virus-Like Particles | Inquiry |
| VLP-N-00010 | Human TRPC4 Virus-Like Particles | Inquiry |
| VLP-N-00011 | Human KCNK1 Virus-Like Particles | Inquiry |
| VLP-N-00012 | Human MS4A1 Virus-Like Particles | Inquiry |
| VLP-N-00013 | Human CLDN1 Virus-Like Particles | Inquiry |
In this study, the research team developed an engineered optimized VLP-engineered nucleocytosolic vehicles for loading of programmable editors (ENVLPE). By introducing GCN4 protein, a more efficient ENVLPE+ was constructed, which can efficiently deliver mRNA, CRISPR-Cas9 (including CRISPRa, CRISPRi), base editors (BE) and prime editors (PE).
1. Intelligent navigation system: nucleocytoplasmic shuttling technology
Traditional VLP can only package editing tools in the cytoplasm, while gene editing needs to be carried out in the nucleus. The research team installed "nuclear localization signal + nuclear export signal" on the carrier protein to achieve autonomous shuttling of the cell nucleus and directly package and transport the gene editor editing.
2. Precision grabbing mechanism: PP7 aptamer lock
Adding PP7 aptamers to the end of gRNA through genetic engineering is like putting a unique barcode on the package. The PP7 binding domain (PCP) on the carrier protein can accurately identify and grab the complete RNP, ensuring that only "assembled" effective editing tools are packaged.
3. Anti-degradation armor: Csy4 protein shield
For the easily degradable pegRNA in the prime editing, the research team introduced the bacterial protein Csy4. It can firmly bind to the RNA end like a "protective cover". Experimental results show that the efficiency of prime editing can be increased by 20 times, successfully solving the long-term technical pain point of insufficient prime editing efficiency.
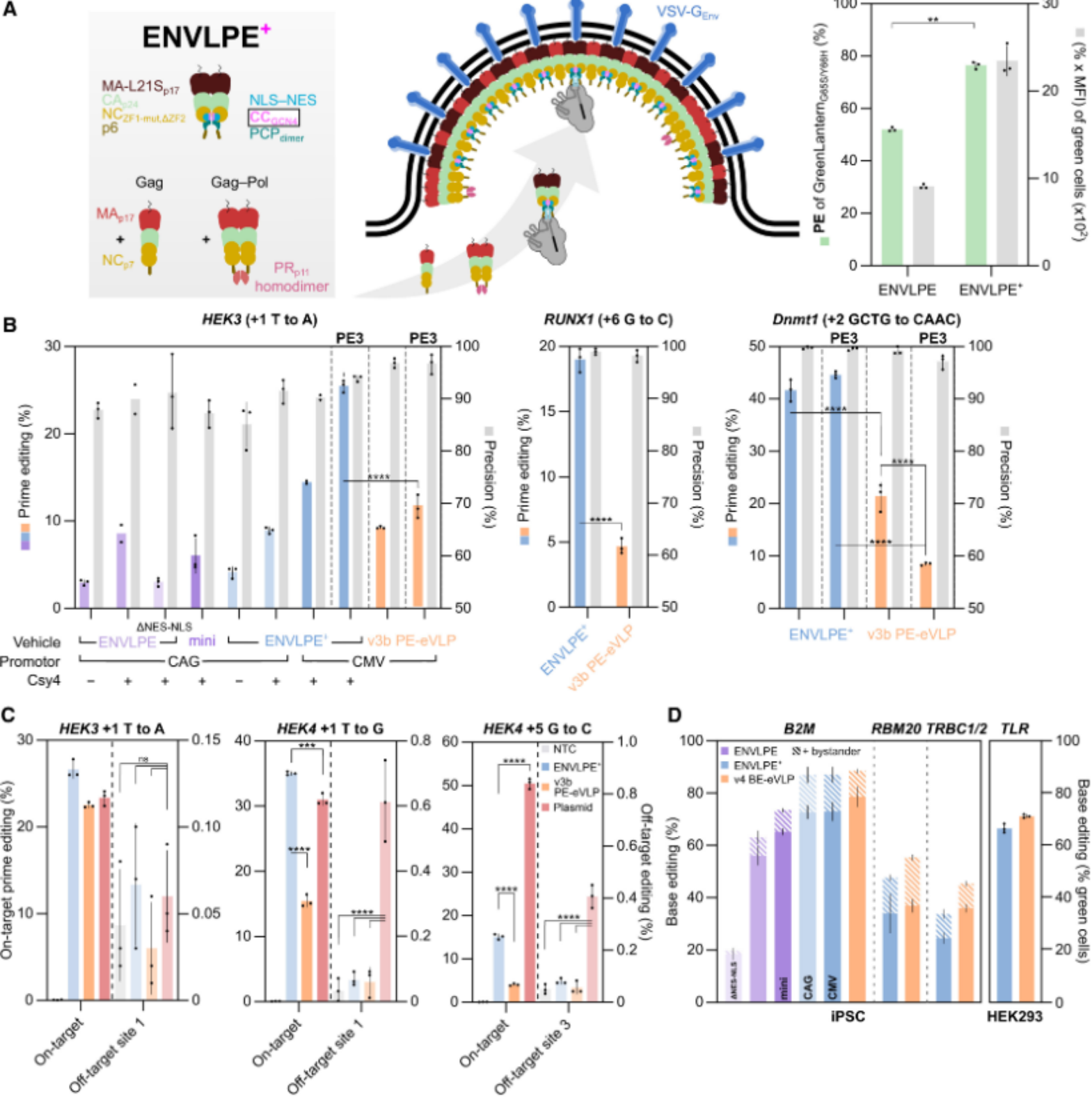
Figure 1. Optimized ENVLPE+ benchmarked for PE and BE at several endogenous sites. (Geilenkeuser J, et al., 2025)
In two inherited retinal disease mouse models, the prime editing delivered by the ENVLPE+ system showed excellent results:
- rd6 model (retinitis pigmentosa): After a single injection, the expression of the key protein MFRP was restored, and electrophysiological detection showed that the light signal response increased by 5.5 times.
- rd12 model (Leber congenital amaurosis): The RPE65 gene mutation was successfully repaired, and 11-cis-retinal (a molecule essential for vision) in the retina was restored to 20% of the normal level.
In addition, the base editors delivered by the ENVLPE+ system achieved double gene knockout (B2M + TRBC1/2) in human T cells with an efficiency of up to 90%, providing a safer and more efficient solution for CAR-T cell therapy.
Reference
Geilenkeuser J, et al. Engineered nucleocytosolic vehicles for loading of programmable editors. Cell, 2025.

