In the battlefield of cancer treatment, immune checkpoint inhibitors (ICI) are like a brave warrior helping immune cells to attack cancer cells more effectively. However, they also face an embarrassing dilemma: among all patients who receive treatment, only less than 20% of them can benefit from it. This means that most patients have to face the cruel reality of ineffective treatment after receiving treatment with hope. This low response rate has undoubtedly cast a shadow on cancer treatment, and it has also made scientists urgently look for new strategies to improve the effect of immunotherapy so that more patients can rekindle the hope of life.
Immunotherapy, especially the method of activating the immune system to attack cancer cells through anti-PD-1 or anti-PD-L1 antibodies, is considered a revolutionary breakthrough in the field of cancer treatment. However, tumor mutation burden (TMB) as a key biomarker for predicting immunotherapy response has exposed obvious limitations in practical applications. Although tumors with high TMB should theoretically respond better to immune checkpoint inhibitors, about 30% of tumors are still classified as "immune desert" tumors, that is, despite the high TMB, the body's immune cells still find it difficult to infiltrate the tumor, which will lead to poor treatment effects. This phenomenon of limited immune cell infiltration makes it difficult for immunotherapy to play its due role in these patients, which greatly limits the widespread application of immunotherapy.
Recently, in a research report entitled "DDX54 downregulation enhances anti-PD1 therapy in immune-desert lung tumors with high tumor mutational burden" published in the international journal Proceedings of the National Academy of Sciences, scientists from the Korea Advanced Institute of Science and Technology and other institutions successfully identified DDX54 as a key regulatory factor in lung cancer cell immune escape by deeply studying the transcriptome and genomic data of patients with immune escape lung cancer and using systems biology technology to infer gene regulatory networks. The results showed that by inhibiting DDX54, the infiltration of immune cells into tumors can be significantly increased, and the effect of immunotherapy can be significantly improved.
| Cat.No. | Product Name | Price |
|---|---|---|
| CSC-DC004165 | Panoply™ Human DDX54 Knockdown Stable Cell Line | Inquiry |
| CSC-SC004165 | Panoply™ Human DDX54 Over-expressing Stable Cell Line | Inquiry |
| AD04718Z | Human DDX54 adenoviral particles | Inquiry |
| LV10513L | human DDX54 (NM_024072) lentivirus particles | Inquiry |
| LV10514L | human DDX54 (NM_001111322) lentivirus particles | Inquiry |
| CDFG004221 | Human DDX54 cDNA Clone(NM_024072.3) | Inquiry |
| MiUTR1M-03763 | DDX54 miRNA 3'UTR clone | Inquiry |
In this study, the researchers found that inhibiting DDX54 significantly increased the infiltration of anti-tumor immune cells (such as T cells and natural killer cells) and greatly improved the response to immunotherapy by studying allogeneic mouse models. Single-cell transcriptomics and spatial transcriptomics analysis further showed that combined treatment with DDX54 promoted the differentiation of T cells and memory T cells, which can inhibit tumor growth while reducing the infiltration of regulatory T cells and exhausted T cells, and they usually promote tumor growth. Mechanistically, inhibiting DDX54 can inactivate signaling pathways such as JAK-STAT, MYC and NF-κB, thereby downregulating the expression of immune escape proteins CD38 and CD47. This not only reduces the infiltration of circulating monocytes that promote tumor development, but also promotes the differentiation of M1 macrophages that play an anti-tumor role.
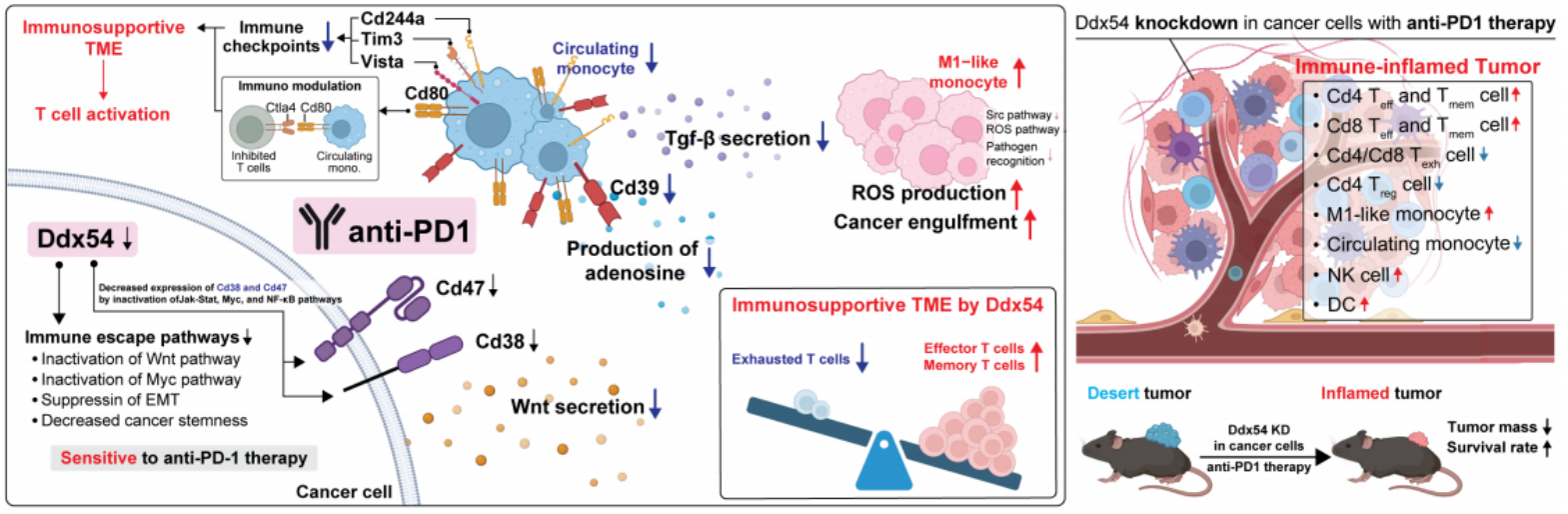
Figure 1. The schematic figure depicts the remodeling of lymphoid and myeloid compartments under Ddx54 knockdown and anti-PD1 treatment, illustrating the conversion of an immune-suppressive TME into an immune-supportive one, thereby enhancing the therapeutic effects of anti-PD1 treatment. (Gong J R, et al., 2025)
This study not only successfully identified a key regulatory factor DDX54 hidden in the complex molecular network of cancer cells, but also combined information technology and biotechnology through a systems biology approach, thus providing a new strategy for human cancer treatment. By targeting DDX54, scientists are expected to develop a new treatment strategy that can make cancers that are resistant to immunotherapy become sensitive again. This research discovery is not only expected to break the bottleneck of immunotherapy, but also bring new hope to patients who do not respond to traditional immunotherapy. In addition, this technology has been transferred to the startup BioRevert Inc and is being developed as a companion therapy drug. It is expected to enter the clinical trial stage in 2028. This progress has undoubtedly brought new hope to cancer patients and injected new vitality into the field of immunotherapy.
Researcher Professor Kwang-Hyun Cho said that this research discovery is not only a major breakthrough in lung cancer immunotherapy, but also provides new ideas for the treatment of patients with other types of cancer. Through the methods of systems biology, we can better understand the complex molecular networks of cancer cells and discover more hidden key regulatory factors, bringing more possibilities for cancer treatment. Finally, the researchers said that the discovery of DDX54 may be just the tip of the iceberg, and there are more unknowns waiting for scientists to uncover in the future.
Reference
Gong J R, et al. DDX54 downregulation enhances anti-PD1 therapy in immune-desert lung tumors with high tumor mutational burden. Proceedings of the National Academy of Sciences, 2025, 122(14): e2412310122.

