In a new study, researchers from the Georgia Institute of Technology have created a two-pronged approach that marks tumor cells so they can be recognized and destroyed by specially enhanced T cells from the patient's immune system. This approach could one day become a universal strategy for treating some of the most difficult-to-treat cancers, such as brain, breast and colon cancers, by teaching the immune system to find cancers it would normally miss. Their approach worked against these cancers in laboratory tests and did not harm healthy tissue. Importantly, it also prevented the cancer from coming back.
While the therapy is still in early development, it builds on proven, safe technology and provides a clearer, faster path to clinical trials and patient care.
In the new study, Gabe Kwong, an associate professor in the Department of Biomedical Engineering at Georgia Tech, and his team found that they could use lipid nanoparticle-based mRNA delivery to express synthetic antigens called camelid single-domain antibodies (VHH) to tag tumors. They then used CAR-T cell therapy to train the body's immune system to seek out this synthetic antigen and destroy the tagged tumor cells.
"Tumor cells are cunning," Kwong said. "Most of the time, immune cells basically can't see them because they come from our own tissues. Normally, if you want to design T cells to target cancer, you need to find the characteristics of each different cancer to design T cells to target that cancer. In this case, we designed CAR-T cells to recognize this synthetic antigen, which becomes a universal platform."
CAR-T cell therapy has been shown to be effective for some cancers and some patients, especially liquid tumors that circulate in the blood, such as leukemia. The patient's own T cells, a type of white blood cell in the immune system, are genetically modified to express a chimeric antigen receptor (CAR) and then delivered back into the patient to seek out and destroy tumors. Unfortunately, healthy cells often suffer collateral damage because they also have the protein that the CAR-T cells are looking for, causing complications for the patient.
In the new study, Kwong's team, led by Lena Gamboa and Ali Zamat, gave the CAR-T cells a clear target that is expressed only on cancer cells and not on any healthy tissue. Their synthetic antigen is such a protein target that is not found in the human body.
The combined approach also showed tenacious vitality: After eliminating the tumor, they reintroduced cancer cells into the animals, and the cancer cells were quickly recognized and attacked. "We turned the tumor into a kind of immune training center, so that if the cancer cells appear again, the body's own natural immune cells can learn to recognize them," Gamboa said.
Marking tumors in this way also provides a way to treat cancers that have no targets that can be attacked by drugs, providing options for patients who don't have many options.
"Triple-negative breast cancer does not express the typical receptor that most breast cancer therapies rely on, leaving patients with few options other than chemotherapy," Zamat said. "Instead of discovering a new drug target, we introduced one and trained the immune system to recognize the cancer in nature."
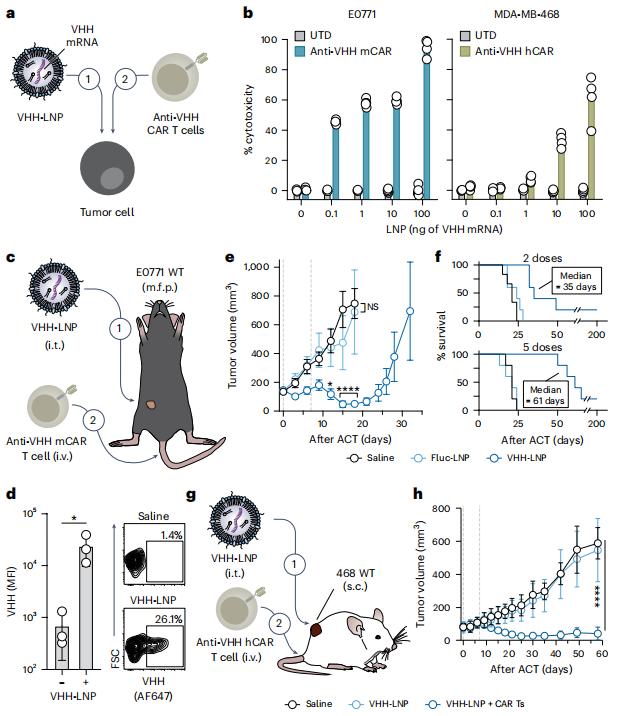
Figure 1. LNP-mediated syntAg delivery sensitizes tumors to CAR-mediated cytoxicity in models of TNBC. (Gamboa L, et al., 2025)
They tested the therapy on breast, brain and colon tumors, demonstrating its effectiveness against a variety of localized solid tumors. These tumors also happen to be cancers known for recurring and sometimes spreading to other parts of the body. Surgery to remove the tumor or radiation therapy usually doesn't cure cancer. At least in the lab, this "mark and attack" strategy was able to cure cancer.
"This is important because it gives us another potential option to control local and regional disease earlier, before it spreads," Kwong said. "Today, there aren't many treatment options. When people think about cancer treatment, they think about the patient who is very sick and has widespread disease. But they never really ask why their disease is widespread. It's because we didn't control the local disease."
There's more research to be done to turn Kwong's team's synthetic antigens into approved cancer treatments. But the team designed the approach with that in mind. Thousands of patients have been treated with other types of CAR-T cell therapies, which have proven to be safe.
Similarly, delivering mRNA to tumor cells via tiny fat bubbles called lipid nanoparticles is a well-known safe approach. "We know the inflammatory spectrum, and we know how many people have received multiple doses of mRNA without any adverse effects," Kwong said. "The same is true for genetically modified CAR-T cells. This makes the path to the clinic very close."
| Cat.No. | Product Name | Price |
|---|---|---|
| CARNP-001 | ADGRE2 CAR mRNA-LNP | Inquiry |
| CARNP-002 | ALK CAR mRNA-LNP | Inquiry |
| CARNP-003 | ARP2/3 CAR mRNA-LNP | Inquiry |
| CARNP-004 | AXIN2 CAR mRNA-LNP | Inquiry |
| CARNP-005 | AXL CAR mRNA-LNP | Inquiry |
| CARNP-006 | B7H3 CAR mRNA-LNP | Inquiry |
| CARNP-007 | B7H6 CAR mRNA-LNP | Inquiry |
| CARNP-008 | BCMA CAR mRNA-LNP | Inquiry |
| CARNP-009 | Biotin CAR mRNA-LNP | Inquiry |
| CARNP-010 | CAIX CAR mRNA-LNP | Inquiry |
| CARNP-011 | CD116 CAR mRNA-LNP | Inquiry |
| CARNP-012 | CD123 CAR mRNA-LNP | Inquiry |
| CARNP-013 | CD13 CAR mRNA-LNP | Inquiry |
| CARNP-014 | CD133 CAR mRNA-LNP | Inquiry |
| CARNP-015 | CD138 CAR mRNA-LNP | Inquiry |
Reference
Gamboa L, et al. Sensitizing solid tumors to CAR-mediated cytotoxicity by lipid nanoparticle delivery of synthetic antigens. Nature Cancer, 2025: 1-15.

