Some anti-cancer therapies not only target tumor cells but also affect healthy cells in the body. If the effect on healthy cells is too strong, the use of this anti-cancer therapy may be limited.
Recently, in a research report titled "Transcription–replication conflicts underlie sensitivity to PARP inhibitors" published in the international journal Nature, scientists from the University of Geneva and other institutions have studied the mechanism of action of PARP inhibitors, especially in the treatment of breast and ovarian cancer patients carrying BRCA gene mutations. They found that the inhibitor blocked two specific activities of the PARP protein. By blocking one of them, the toxic effect on cancer cells can be maintained without affecting the function of healthy cells. Researchers aim to help improve the therapeutic efficacy of these therapies.
Although thousands of injuries damage our DNA every day, the genome in our cells is particularly stable thanks to efficient repair systems. Among the genes encoding repair proteins are BRCA1 and BRCA2, which are involved in the breakage of the DNA double helix. The presence of mutations in these genes (which occur in approximately 2 out of every 1,000 women) results in damaged DNA that cannot be repaired, significantly increasing the risk of breast and ovarian cancer. For men, it increases the risk of prostate cancer.
PARP inhibitors have been used to treat these types of cancer for about 15 years. PARP proteins can detect breaks or abnormal structures in the DNA double helix. It then briefly attaches to DNA and synthesizes a sugar chain, which serves as an alarm signal to recruit proteins involved in DNA repair.
Therapies based on PARP inhibitors block these activities and trap the PAPR protein on DNA so that there are no alarm signals that trigger DNA repair. However, this therapy has proven to be toxic to fast-growing cells such as cancer cells, which produce too many mutations and do not have time to repair them, so they are destined to die. But our bodies are also host to fast-growing healthy cells, such as hematopoietic stem cells (the source of red and white blood cells), which can act as collateral victims and can be decimated by anti-PARP therapies. Researchers currently do not understand the molecular mechanisms behind anti-PAPR drugs killing cells (cancerous or non-cancerous).
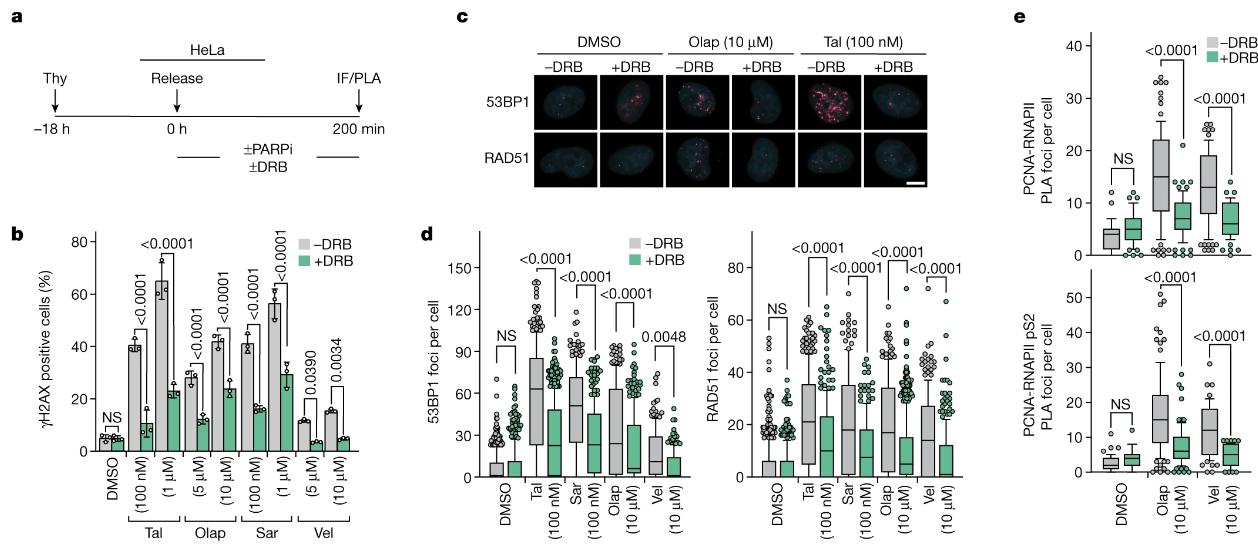
Figure 1. PARP inhibitors induce TRCs. (Petropoulos M, et al., 2024)
In this study, researchers Thanos Halazonetis and others analyzed the molecular mechanism by which PAPR inhibitors work. They used two types of PARP inhibitors that also block PAPR's enzymatic activity (the synthesis of sugar chains that serve as alarm signals) but do not trap PAPR on DNA with the same strength. The researchers then observed that the two inhibitors killed cancer cells with the same efficiency, but the inhibitor that weakly bound PAPR to DNA was much less toxic to healthy cells.
"We found that PARP not only acts as an alarm signal to recruit DNA repair proteins, but also intervenes when different machines that read or copy the same DNA parts collide to form abnormal DNA structures." said Michalis Petropoulos, "This collision prevention warning signal is not triggered when anti-PARP therapy is used. Collisions between these machines lead to increased levels of DNA damage that are not repaired in cancer cells because they lack the BRCA repair protein."
The second activity of PARP therapy causes PARP to bind (trap) tightly to DNA, causing DNA damage that needs to be repaired by the cell. But this repair is not mediated by BRCA repair proteins, so both normal cells and cancer cells are killed. The researchers found that inhibition of the enzyme's activity may be enough to kill cancer cells, but that when PAPR binds strongly to DNA, this capture also kills normal cells. And this may be caused by the toxicity of these drugs. The findings may help researchers develop safer PAPR inhibitors that inhibit PARP's enzyme activity without trapping it on DNA. Taken together, the results of this study suggest that inhibiting the enzymatic activity of PAPR may be sufficient to achieve therapeutic efficacy in the absence of homologous recombination.
Reference
Petropoulos M, et al. Transcription–replication conflicts underlie sensitivity to PARP inhibitors. Nature, 2024: 1-9.