Recently, the U.S. Food and Drug Administration (FDA) officially approved a groundbreaking medical trial - using gene-edited pig livers to treat patients with liver failure! Patients with severe liver failure who cannot receive human organ transplants are temporarily connected to pig livers outside the body, which filter their blood. This is a huge breakthrough in the field of xenotransplantation, and it also brings new hope of survival to countless patients waiting for organ transplants.
The mortality rate of patients with liver failure is as high as 50%, and many patients die because they cannot wait for human liver sources. This clinical trial, led by the US biotechnology company eGenesis and the UK OrganOx, plans to install "ex vivo pig livers" for 4 critically ill liver failure patients who cannot receive human liver transplants. Through the external gene-edited pig liver, blood flows through the pig liver to filter toxins, maintaining life for up to 72 hours, giving patients time to recover liver function or wait for organ transplants.
The subjects of the trial are patients with acute and chronic liver failure aged 10-70 years, who will be monitored for up to 1 year after surgery. If the early data is safe, the trial will be further expanded to 20 people. Scientists hope that this "transitional treatment" can become a "life-saving bridge" for patients with liver failure in the future.
Pig organs are close in size to humans, but direct transplantation can cause strong immune rejection. In addition, the porcine retrovirus genes present in the pig genome may pose risks after xenotransplantation.
CRISPR gene editing technology enables us to solve the above obstacles more accurately and efficiently. Based on the research of George Church, Yang Luhan and others, eGenesis has created gene-edited pigs that can solve the two major obstacles of immune rejection and porcine retrovirus, thereby providing human-compatible organs and cells to solve the transplant donor shortage crisis.
In October 2023, eGenesis published a paper in the journal Nature, using CRISPR gene editing technology to perform a record 69 gene edits on Yucatan mini pigs, knocking out three genes expressing glycan antigens that would cause immune rejection (GGTA1, CMAH, and B4GALNT2L), and knocking in seven human genes to reduce the immune system's resistance (these seven human genes include complement-related CD46 and CD55, coagulation-related THBD and PROCR, innate immunity-related CD47, TNFAIP3 and HMOX1 that inhibit ischemia-reperfusion injury, apoptosis, and inflammation). The other 59 gene edits inactivated all copies of porcine retroviral genes.
In January 2024, eGenesis and OrganOx announced the world's first ex vivo perfusion of a gene-edited pig liver into a brain-dead patient. The patient in the study died of brain hemorrhage, and his family generously donated to participate in the study while his heart was still beating. The research team used a gene-edited pig liver developed by eGenesis and connected it to OrganOx's ex vivo liver cross-circulation (ELC) device to circulate the donor's blood through the gene-edited pig liver (the patient's own liver remained in the body). Stable blood flow, pressure, and pH were maintained throughout the perfusion process, with strong bile production, and no rejection was observed. After 72 hours of continuous perfusion, the research team chose to stop the study as planned, and the liver still looked healthy.
In March 2025, researchers from the Xijing Hospital of the Chinese Air Force Medical University published a paper in the journal Nature, describing the case of a gene-edited pig liver transplanted into a human recipient diagnosed with brain death. The organ successfully survived and functioned normally for 10 days in a brain-dead patient. The research team monitored the function, blood flow, and immune and inflammatory responses of the pig livers over a 10-day period. The results showed that the pig livers functioned normally in the human body and produced bile and porcine serum albumin, maintained stable blood flow, and showed no signs of rejection. These results indicate that genetically modified pig livers can survive and function in the human body and are expected to serve as a transitional therapy for patients with liver failure waiting for human donors.
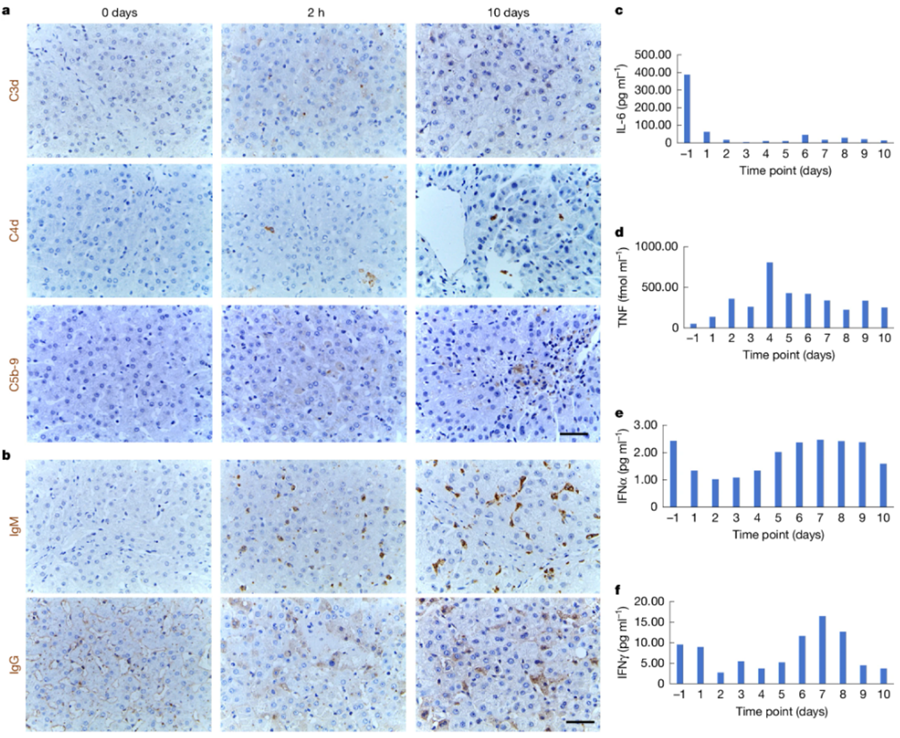
Figure 1. Immune and inflammatory monitoring of the recipient. (Tao K S, et al., 2025)
In general, the above cases and the approval of clinical trials are a huge leap forward in the field of xenotransplantation! Decades of research have proven that animal organs (pig organs) may change the dilemma of organ shortage. We can predict that in the future, pig organs (pig liver, pig heart, pig kidney) may help patients maintain their lives while waiting for a transplant, or even restore organ function.
References
Anand R P, et al. Design and testing of a humanized porcine donor for xenotransplantation. Nature, 2023, 622(7982): 393-401.
Tao K S, et al. Gene-modified pig-to-human liver xenotransplantation. Nature, 2025: 1-8.

