In the frontier field of medical research, skin wound healing has always been the focus of scientists. Whether it is accidental trauma, surgical incision, or skin ulcer caused by chronic disease, the speed and quality of wound healing directly affect the patient's recovery process. However, there are many complex molecular mechanisms in the skin repair process, some of which have not yet been fully revealed.
Recently, in a research report entitled "Collaborative Duality of CircGLIS3(2) RNA and Protein in Human Wound Repair" published in the international journal Advanced Science, scientists from the Karolinska Institute and other institutions in Sweden revealed the unique role of a circular RNA called CircGLIS3(2) in skin wound healing. This discovery is expected to bring new therapeutic strategies to improve wound healing and reduce scar formation.
The skin is the largest organ in the human body. It not only protects the body from external damage, but also participates in multiple physiological functions such as regulating body temperature and sensing external stimuli. When the skin is damaged, the body will quickly initiate a series of complex repair mechanisms. The wound healing process is roughly divided into the inflammatory phase, the proliferation phase, and the remodeling phase. During the inflammatory phase, immune cells gather at the wound to clear pathogens and necrotic tissue. During the proliferative phase, skin cells begin to proliferate and migrate to fill the wound. Finally, during the remodeling phase, the new tissue gradually matures and restores the normal structure and function of the skin. However, this process does not always go smoothly. In some cases, wound healing may be abnormal, such as healing too slowly and forming excessive scars (such as keloids). These abnormalities not only affect the patient's appearance, but may also cause functional disorders and cause great pain to the patient. Therefore, a deep understanding of the molecular mechanisms of skin wound healing is of great significance for the development of more effective treatments.
Here, the researchers first established a unique human skin wound healing model. By sampling full-thickness skin incision wounds from healthy volunteers and collecting wound tissues at 1 day, 7 days, and 30 days after injury, the researchers captured changes in gene expression during the three stages of inflammation, proliferation, and remodeling. By performing RNA sequencing analysis on wound tissues and isolated skin cells, the researchers found that the expression level of a circular RNA called CircGLIS3(2) was significantly increased in wound fibroblasts. Fibroblasts are a key cell type in the skin repair process that can synthesize extracellular matrix (ECM), promote wound contraction, and secrete growth factors and cytokines to communicate with other cells. This expression change of CircGLIS3(2) suggests that it may play an important role in wound healing.
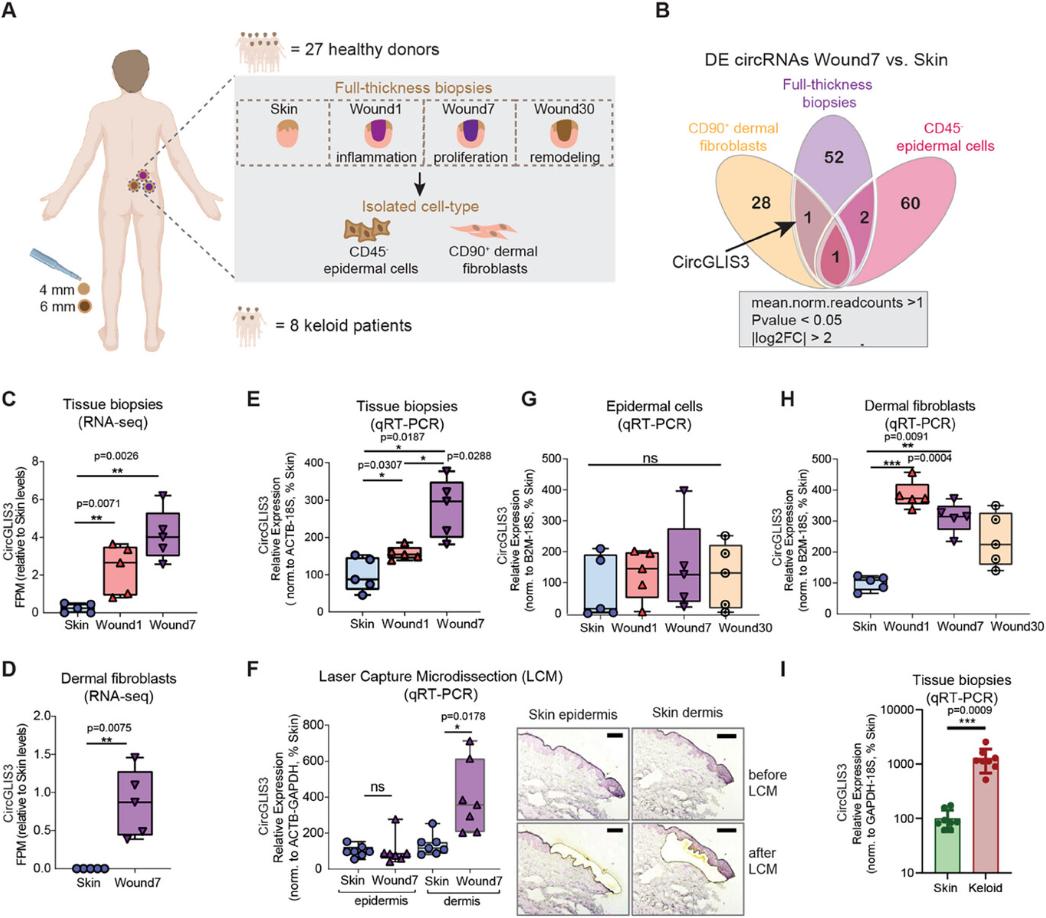
Figure 1. CircGLIS3(2) expression is upregulated in human wound fibroblasts. (Niu G, et al., 2025)
Further molecular characterization analysis showed that CircGLIS3(2) is derived from the second exon of the GLIS3 gene and is a conserved circular RNA. It is mainly located in the cytoplasm of fibroblasts and has a long half-life (22 hours), which allows it to remain stable in cells for a long time and thus better perform its functions. The researchers also found that the expression of CircGLIS3(2) is induced by a variety of wound-related signals, such as interleukin-1α (IL-1α), transforming growth factor-β (TGF-β), hypoxia and endoplasmic reticulum stress. These signals appear in the early stages of wound healing, suggesting that the upregulation of CircGLIS3(2) may be a rapid response to the wound environment.
The most exciting discovery is that the researchers discovered the "dual-sided" function of CircGLIS3(2). On the one hand, as an RNA, CircGLIS3(2) can stabilize the PCOLCE protein in the cytoplasm. The PCOLCE protein plays an important role in the maturation of collagen. It can help remove C-propeptide from procollagen by promoting the activity of bone morphogenetic protein 1/like protease, which is a key step in collagen fibril formation. The researchers also confirmed the interaction between CircGLIS3(2) and PCOLCE protein through a series of experiments (including RNA binding protein immunoprecipitation (RIP) and fluorescence in situ hybridization (FISH) techniques) and found that CircGLIS3(2) can enhance the contractile ability of fibroblasts and the production of extracellular matrix by stabilizing PCOLCE protein.
On the other hand, CircGLIS3(2) can also encode a 131-amino acid protein that can bind to BTF3 protein in the cell nucleus and promote cell proliferation by interacting with BTF3. BTF3 is a transcription factor that plays an important role in the regulation of the cell cycle and can promote the transition of cells from G1 phase to S phase, thereby promoting cell proliferation. The researchers confirmed the interaction between CircGLIS3(2) protein and BTF3 through co-immunoprecipitation (co-IP) and immunofluorescence co-staining experiments, and found that CircGLIS3(2) protein can promote the proliferation of fibroblasts by stabilizing BTF3 protein.
This study revealed the unique "dual-faced" function of CircGLIS3(2) in skin wound healing. Previous studies usually regarded circRNA as non-coding RNA, focusing mainly on its indirect role in gene expression regulation. However, CircGLIS3(2) can not only function as RNA, but also encode proteins. This dual function enables it to promote the production of extracellular matrix and cell proliferation simultaneously during wound healing. This "two-in-one" feature not only demonstrates the versatility of circRNA in cell physiological regulation, but also provides new ideas for future gene therapy. By regulating the expression of CircGLIS3(2), it may be possible to simultaneously promote the speed and quality of wound healing and reduce scar formation.
| Cat.No. | Product Name | Price |
|---|---|---|
| PMCR-0001 | EGFP circRNA | Inquiry |
| PMCR-0002 | Firefly Luciferase circRNA | Inquiry |
| PMCR-0003 | Gaussia Luciferase circRNA | Inquiry |
| PMCR-0004 | Renilla Luciferase circRNA | Inquiry |
| PMCR-0005 | mCherry circRNA | Inquiry |
| PMCR-0006 | β-galactosidase circRNA | Inquiry |
| PMCR-0007 | Luciferase P2A GFP circRNA | Inquiry |
| PMCR-0008 | Cas9 circRNA | Inquiry |
| PMCR-0009 | NLS-Cre circRNA | Inquiry |
| PMCR-0010 | Cas9 Nickase circRNA | Inquiry |
| PMCR-0011 | Cas9-T2A-EGFP circRNA | Inquiry |
| PMCR-0012 | Cre-T2A-EGFP circRNA | Inquiry |
| PMCR-0013 | OVA circRNA | Inquiry |
| PMCR-0014 | EPO circRNA | Inquiry |
Reference
Niu G, et al. Collaborative Duality of CircGLIS3 (2) RNA and Protein in human Wound Repair. Advanced Science, 2025: 2416784.

