Around the world, about 162,000 people are struggling with cystic fibrosis (CF), a serious genetic disease. CF is an autosomal recessive genetic disease that severely shortens the lifespan of patients. It is caused by loss-of-function mutations in the CF transmembrane conductance regulator (CFTR) gene. These mutations affect the synthesis, folding, transport, and gating properties of the CFTR anion channel, thereby interfering with the transport of chloride and bicarbonate ions. Although modulatory drugs have made some progress in the treatment of CF, existing modulatory drugs are powerless for patients carrying specific mutations, such as those with the G542X mutation. Recently, a study published in iScience titled "Adenine base editing with engineered virus-like particles rescues the CFTR mutation G542X in patient-derived intestinal organoids" has brought new hope.
| Cat.No. | Product Name | Price |
|---|---|---|
| CSC-DC003043 | Panoply™ Human CFTR Knockdown Stable Cell Line | Inquiry |
| CSC-RI0003 | Human CFTR Stable Cell Line-HEK293 | Inquiry |
| CSC-RI0081 | Human CFTR Stable Cell Line-CHO | Inquiry |
| CSC-RK0155 | Cftr Knockout Mouse Lung Endothelial Cells | Inquiry |
| CSC-RK0156 | Cftr Knockout Mouse Kidney Epithelial Cells | Inquiry |
| CSC-RK0157 | Cftr Knockout Mouse Heart Fibroblasts | Inquiry |
| CSC-RK0158 | Cftr Knockout Mouse Spleen Fibroblasts | Inquiry |
| CSC-RK0159 | Cftr Knockout Mouse Liver Fibroblasts | Inquiry |
| CSC-RK0160 | Cftr Knockout Mouse Kidney Fibroblasts | Inquiry |
| CSC-RK0161 | Cftr Knockout Mouse Lung Fibroblasts | Inquiry |
| CSC-RK0164 | Cftr Knockout Mouse Bone Marrow Macrophages | Inquiry |
The study focused on the G542X mutation, which is the second most common pathogenic mutation in CF. It is caused by the c.1624G>T mutation, which leads to the appearance of premature termination codons (PTC), triggering nonsense-mediated mRNA decay (NMD), making the CFTR protein carrying this mutation unable to function normally and unresponsive to existing modulatory drugs. Although previous CFTR gene editing studies have achieved results, they have their own limitations. Base editing technology has attracted much attention among many editing strategies because it can achieve precise single nucleotide editing and does not involve double-strand break formation.
In this study, the research team used engineered virus-like particles (BE-eVLPs) to deliver NG-ABE8e/sgRNA ribonucleoproteins (RNPs) to patient-derived intestinal organoids. Attempts were made to edit the G542X mutation into the non-pathogenic G542R mutation to restore CFTR function.
The results of the study are exciting. First, NG-ABE8e successfully edited G542X to G542R, and the editing efficiency averaged 2%. When testing intestinal organoids from two donors, it was found that editing from G542X to G542R occurred in organoid cells transduced with BE-eVLPs (G542X-BE-eVLPs) carrying NG-ABE8e and G542X-specific sgRNAs. In contrast, DNA editing did not occur in cells transduced with BE-eVLPs without sgRNA.
Secondly, the editing process is highly accurate. No editing of potential bystander sites was detected above background levels within the 20-nt long spacer binding region, indicating that the editing design was extremely accurate and effectively avoided unnecessary mutations. At the same time, no insertion or deletion (indel) formation was detected by CRISPResso2 analysis. In terms of off-target effects, the researchers used CRISPOR for computer analysis and found eight exon off-target sites. After amplification and sequencing of the ADAMTS6 site, which was most likely to undergo off-target editing, it was found that the editing efficiency was not higher than the background level. Other sites have a low probability of off-target editing due to mismatches in the seed region.
Furthermore, the presence of G542R effectively restored the activity of CFTR. When the transduced intestinal organoids were functionally tested, it was found that G542X-BE-eVLPs successfully rescued the activity of CFTR. As measured by short-circuit current (DIsc), 2% G542X to G542R editing restored 6.4% of wild-type CFTR activity, and the variability between organoids from the two donors was negligible. Similar results were obtained in the forskolin-induced swelling (FIS) experiment conducted in 3D cultured organoids. Organoids transduced with G542X-BE-eVLPs responded significantly to FIS, up to 16% of the total unsorted organoids, and the area of these organoids increased significantly after stimulation. In addition, the study found that the addition of a modulating drug combination (Elexacaftor, Tezacaftor, and Ivacaftor) had no additional effect on G542R function.
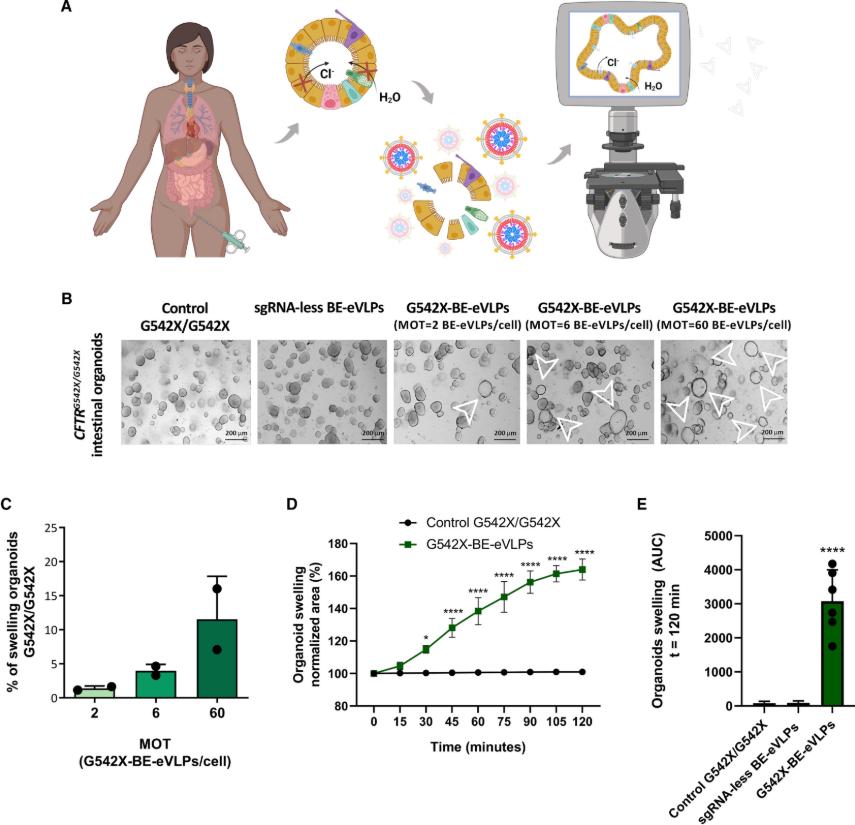
Figure 1. G542X-BE-eVLPs restore FIS in 3D-grown patient-derived intestinal organoids. (Nicosia L, et al., 2025)
In general, this study used adenine base editing technology to successfully rescue the G542X mutation in patient-derived intestinal organoids, opening up a new avenue for the treatment of cystic fibrosis. Although it is still in the proof-of-principle stage, the results fully demonstrate the great potential of base editing technology in correcting pathogenic mutations and lay a solid foundation for future in vivo applications.
Reference
Nicosia L, et al. Adenine base editing with engineered virus-like particles rescues the CFTR mutation G542X in patient-derived intestinal organoids. iScience, 2025, 28(3).

