
Our promise to you:
Guaranteed product quality, expert customer support.
 24x7 CUSTOMER SERVICE
24x7 CUSTOMER SERVICE
 CONTACT US TO ORDER
CONTACT US TO ORDER
CRISPR-Cas9 Genome Engineering: Treating Inherited Retinal Diseases 
Gene editing technology can be regarded as a molecular tweezer guiding targeted removal or correction of disease causing genetic mutations. Inherited retinal diseases (iRDs) comprise an extensive collection of heterogeneous mutations in more than 250 genes, leading to syndromic or non-syndromic sight loss. At present, available therapeutic approaches for retinal diseases (RD) include nutritional supplements, neurotrophic factors, antiangiogenic drugs for wet AMD and gene augmentation/interference strategy for IRDs. However, these therapies do not aim at correcting the genetic defect, resulting in inefficient and expensive treatments. Gene editing technology, particularly CRISPR-Cas9, provides exciting opportunities to precisely replace genetic mutations causative of disease.
Overview of iRDs
iRDs are clinically and genetically heterogeneous in nature, where monogenic disorders can be syndromic or non-syndromic forms and disease-causing gene mutations can be recessive, dominant, or X-linked. iRDs are usually caused by gene mutations, which perturb the development, function, and survival of photoreceptor or retinal pigment epithelial (RPE) cells in the retina. Both recessive and dominant iRD mutations can result in a variety of biological outcomes including, but not limited to, incorrect transport and aggregation of outer segment proteins activating cell stress responses, defective regeneration of visual pigments for phototransduction, or loss of photoreceptor-specific gene expression. So far, the most clinically advanced genetic therapies for IRDs use a gene augmentation strategy for recessive diseases in which an expression cassette for wild-type (WT) cDNA of the mutated gene is packaged in an adeno-associated viral (AAV) vector or lentiviral (LV) vector delivered to the retina. Currently, there are at least 10 different gene augmentation therapies in clinical trials and are rapidly advancing. However, gene augmentation therapy providing a normal copy of a mutated gene into native cells is applicable only for the treatment of haploinsufficiency or loss-of-function mutations and does not directly affect the pathogenic host gene. By comparison, a genome editing method has the potential of correcting the mutation directly in the patient's DNA or ablating the expression of dominant mutations filling the void left by gene augmentation therapy.
Application of CRISPR/Cas9 in Inherited Retinal Diseases
Gene editing technology, particularly CRISPR/Cas9, provides exciting opportunities to precisely replace genetic mutations causative of disease. CRISPR/Cas9 technology has attracted more and more attention and has been widely used in generating knockout cell lines and animal models to mimic diseases. At the same time, it has been widely used for studying gene therapy for a great number of diseases, including retinal diseases. Emerging treatments include ex vivo gene correction in patient-derived, induced pluripotent stem cells (iPSCs) followed by transplantation of retinal progenitor cells into the eye. An alternative in vivo method would employ corrective gene editing directly in the patient's eye. No doubt, versatile, customized gene correction is highly desirable to treat the heterogeneous array of disease-causing gene mutations in iRD.
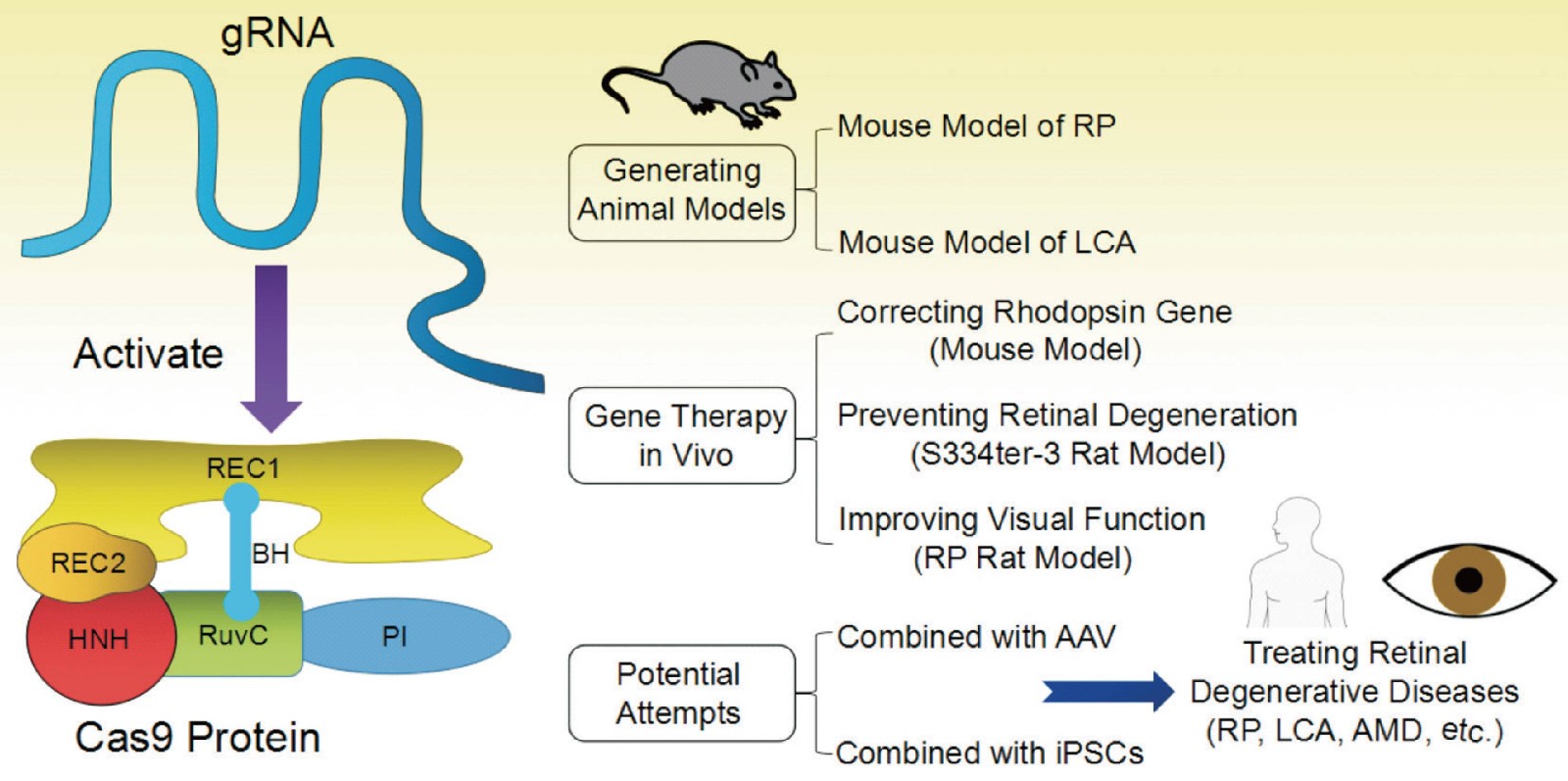 Figure 1. Structure and applications of CRISPR/Cas9 in retinal degenerative diseases. (Peng Y Q, et al., 2017)
Figure 1. Structure and applications of CRISPR/Cas9 in retinal degenerative diseases. (Peng Y Q, et al., 2017)
- Ex vivo gene editing for the treatment of iRDs
Transplantation of gene edited ES or iPSC cells to the retina is a potential therapeutic strategy for iRD. Recently, Bassuk et al. demonstrated ex vivo correction of an X-linked retinitis pigmentosa GTPase regulator (RPGR) iRD mutation using CRISPR/Cas9. This proof-of-concept study shows the capability to repair RPGR ORF15 region by using CRISPR/Cas9. If these findings are safely and effectively applied to the eyes of patients, the treatment will reach a milestone. Despite the applicability of ex vivo genome editing for iRDs, there are still fundamental safety and efficacy issues with downstream cell transplantation. Unfortunately, repairing severely degenerated retinas by cellular transplantation of photoreceptor progenitors suffered a setback. Despite setbacks for photoreceptors, transplantation of autologous or allogenic retinal pigment epithelial (RPE) grafts is a viable treatment option for specific iRDs. Indeed, clinical trials are evaluating the safety and efficacy of RPE cell transplantation. Schwartz et al. conducted two prospective Phase I/II studies to assess tolerability and safety of hESC-derived RPE transplantation in Stargardt's macular dystrophy, and atrophic AMD. Adverse events were related to surgery and immunosuppression, and no adverse proliferation, rejection, or serious ocular or systemic safety issues arose from the transplanted tissue.
- In vivo gene editing for the treatment of iRDs
Advances in delivery approaches developed for gene replacement have accelerated the preclinical investigations of endonucleases as an in vivo gene editing therapy for iRDs. Hung et al. proved CRISPR/Cas9 to effectively knockout yellow fluorescent protein (YFP) in a Thy1-YFP transgenic mouse retina using intravitreal delivery of an AAV2-encapsulated Strep. pyogenes Cas9 (SpCas9) and a single guide RNA (sgRNA) against YFP. This work showed ability to efficiently achieve targeted gene editing knockouts in mammalian retinae. Moreover, the mice maintained visual function 5 weeks after injection, an important proof-of-concept for viral-mediated retinal gene editing in vivo. Bakondi et al. demonstrated subretinal injection of a targeted gRNA/Cas9 plasmid in combination with electroporation generated allele-specific rhodopsin disruption in a rat RP model. In autosomal-dominant disease, allele-specific ablation using gene editing could restore retinal function by the activity of the remaining wild-type allele. The transgenic S334ter rat displays similar phenotypes to the human class I RHO mis-trafficking mutations. Remarkably, CRISPR/Cas9 could selectively disrupt RhoS334 owing to a PAM motif present in RhoS334 but not in RhoWT alleles. Retention of photoreceptor cell number, improved visual acuity, or outer nuclear layer thickness compared to a control gRNA demonstrate the first effective use of retinal gene editing to target iRD mutations in vivo.
Our CRISPR/Cas9 System Services
CRISPR/Cas9 PlatformCB is committed to providing the most professional and comprehensive genetic editing technology solutions for our clients. To support your projects, we offer a comprehensive custom CRISPR/Cas9 gene editing service from strategy design to final iRDs model generation.
➢ Human Cell Models Generation of iRDs by CRISPR/Cas9 System
➢ Animal Models Generation of iRDs by CRISPR/Cas9 System
If you have any questions, please feel free to contact us.
Related Services and Products
Animal Models
- Conditional Knockout Mouse
- Conventional Knockout Mouse
- Point Mutation Mouse
- CRISPR/Cas9 Knockin Mouse
- Rosa26 Knockin Mouse
Cell Lines
- Point Mutation Cell Line Generation
- HIEF™ Site-Specific Knock-in Cell Line Service
- Fragment Deleted Stable Cell Line
- Gene Editing in Primary T Cells
- Gene Knockout Cell Line Generation
Products
- CRISPR/Cas9 Kits
- Pre-made Knockout Cell Line
- CRISPR Lentiviral Library
- Cas9 Related Plasmids
- Pre-made Cas9 Virus Particles
References
- Burnight E R, et al. CRISPR-Cas9 genome engineering: Treating inherited retinal degeneration. Progress in retinal and eye research, 2018, 65: 28-49.
- Benati D, et al. Gene editing prospects for treating inherited retinal diseases. Journal of medical genetics, 2020, 57(7): 437-444.
- Smith A J, et al. Genome editing: the breakthrough technology for inherited retinal disease?. Expert Opinion on Biological Therapy, 2017, 17(10): 1245-1254.
- Peng Y Q, et al. Applications of CRISPR/Cas9 in retinal degenerative diseases. International journal of ophthalmology, 2017, 10(4): 646.
- Vázquez-Domínguez I, et al. Molecular therapies for inherited retinal diseases—Current standing, opportunities and challenges. Genes, 2019, 10(9): 654.