
Our promise to you:
Guaranteed product quality, expert customer support.
 24x7 CUSTOMER SERVICE
24x7 CUSTOMER SERVICE
 CONTACT US TO ORDER
CONTACT US TO ORDER
CRISPR/Cas9 Genome Editing to Treat Hemophilia 
Hemophilia is an X-linked recessive bleeding disorder resulting in spontaneous bleeding and bleeding following trauma and surgery. Although usually expressed in males, female genetic carriers may have clinical bleeding symptoms and even factor activity levels within the hemophilia range. It is characterized by the congenital deficiency of coagulation factor VIII (FVIII; hemophilia A) and factor IX (FIX; hemophilia B). Hemophilia arises from mutations in the F8 and F9 genes, and more than 30% of cases are caused by spontaneous mutations, affecting all racial and ethnic groups. Without the availability of replacement therapy, individuals with severe disease are at risk for recurrent bleeding into joints, soft tissues, muscles, and other locations that can be life threatening (such as central nervous system). Long-term sequelae as a result of recurrent bleeding include chronic pain, chronic arthropathy, muscle atrophy, and loss of mobility with significant disability.
Genome Editing to Treat Hemophilia
As hemophilia therapy has evolved from protein substitution to gene replacement, the next natural step would be gene correction. Now, this has become a reality through fundamental discoveries and engineering breakthroughs, which have produced a toolkit of reagents for genome editing. The four basic platforms are engineered meganucleases, zinc finger nucleases (ZFNs), transcription activator like effector nucleases (TALENs), and clustered regularly interspaced short palindromic repeat (CRISPR)/Cas9 nucleases. The basic premise of these technologies is to introduce a site-specific DNA double-stranded break (DSB) and then enable the cell's own endogenous repair machinery to repair the break. Two major repair pathways are homology-directed repair (HDR) and nonhomologous end joining (NHEJ).
These technologies are being adopted actively for ex vivo or in vivo studies correcting mutated genes that lead to inherited monogenic diseases, such as hemophilia. In particular, the development of a delivery system for in vivo genome-editing which does not require ex vivo manipulation step is actively in progress. According to Li et al., nuclease-mediated in vivo editing utilizing AAV vector delivery system can be made in a tissue specific manner. To date, the AAV vector is being considered as a more promising delivery tool for in vivo editing.
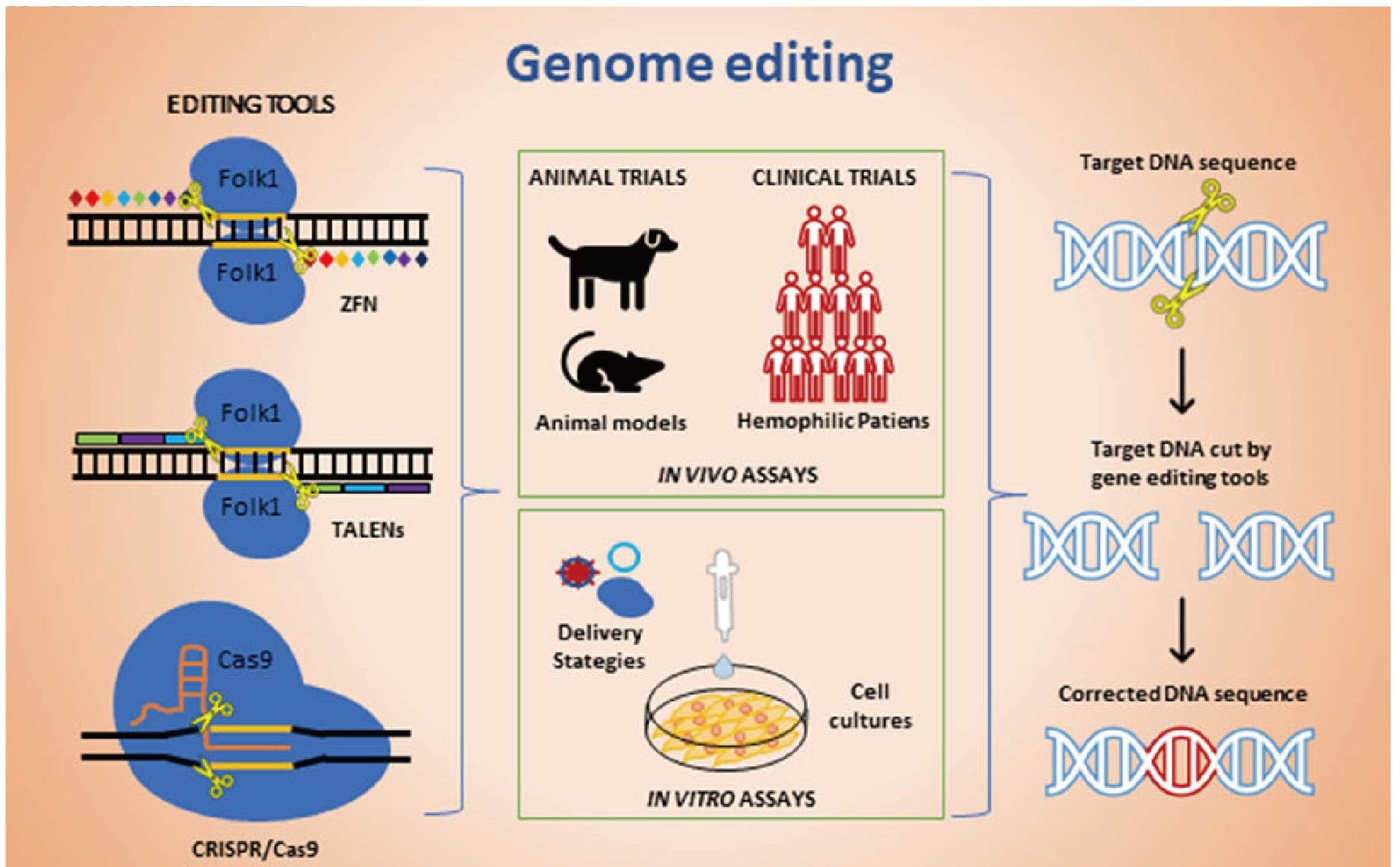 Figure 1. Genome editing therapy strategies for hemophilia. (Robles-Rodríguez O A, et al., 2020)
Figure 1. Genome editing therapy strategies for hemophilia. (Robles-Rodríguez O A, et al., 2020)
Application of CRISPR/ Cas9 in Hemophilia
Several studies have reported successful in vitro correction of the mutations that cause hemophilia A. With the CRISPR/Cas9 system, the intergenic inversion involving the intron 22 (600 kbp) of the FVIII gene was corrected in induced pluripotential stem cells (iPSCs) that are induced from cells isolated from the urine of hemophilia A patients. The use of somatic cells isolated from urine is a safe method for obtaining somatic cells from hemophilia patients as this method does not represent a condition-related risk. These cells can be induced by transcription factors to form pluripotent stem cells and can differentiate into cell lines from the three germinal layers.
The CRISPR/Cas9 system has also been used to correct different mutations that cause hemophilia B. When hemophilia severity associated with each of the mutations in murine models was compared, it was found that a specific mutation (Y371D) causes more severe hemophilia B than other mutations. This murine model was then treated with different adenoviruses, naked DNA expressing the CRISPR/Cas9 system, and factor IX to correct the mutation in hepatocytes. The mice treated with the adenoviruses presented a higher degree of mutation correction but did not show a better therapeutic effect owing to the adenovirus-associated hepatic toxicity. Administration of an adeno-associated virus expressing Cas9 and guide RNA to wild type adult mice achieved the rupture of FIX gene in hepatocytes, which was enough to produce hemophilia B. Afterwards with the same vector, it was possible to insert the FIX cDNA by HDR and NHEJ in one of the introns of the FIX mutated in hepatocytes, reestablishing the production of FIX and correcting hemophilia B.
Our CRISPR/Cas9 System Services
CRISPR/Cas9 PlatformCB is committed to providing the most professional and comprehensive genetic editing technology solutions for our clients. To support your projects, we offer a comprehensive custom CRISPR/Cas9 gene editing service from strategy design to final hemophilia model generation.
➢ Human Cell Models Generation of Hemophilia by CRISPR/Cas9 System
➢ Animal Models Generation of Hemophilia by CRISPR/Cas9 System
If you have any questions, please feel free to contact us.
Related Services and Products
Animal Models
- Conditional Knockout Mouse
- Conventional Knockout Mouse
- Point Mutation Mouse
- CRISPR/Cas9 Knockin Mouse
- Rosa26 Knockin Mouse
Cell Lines
- Point Mutation Cell Line Generation
- HIEF™ Site-Specific Knock-in Cell Line Service
- Fragment Deleted Stable Cell Line
- Gene Editing in Primary T Cells
- Gene Knockout Cell Line Generation
Products
- CRISPR/Cas9 Kits
- Pre-made Knockout Cell Line
- CRISPR Lentiviral Library
- Cas9 Related Plasmids
- Pre-made Cas9 Virus Particles
References
- Pipe S W. Gene therapy for hemophilia. Pediatric blood & cancer, 2018, 65(2): e26865.
- Robles-Rodríguez O A, et al. Advances in gene therapy for hemophilia. Journal of Biosciences, 2020, 45(1): 1-8.
- Park C Y, et al. Genome-editing technologies for gene correction of hemophilia. Human genetics, 2016, 135(9): 977-981.
- Batty P, Lillicrap D. Advances and challenges for hemophilia gene therapy. Human molecular genetics, 2019, 28(R1): R95-R101.
- Ohmori T. Advances in gene therapy for hemophilia: basis, current status, and future perspectives. International journal of hematology, 2020, 111(1): 31-41.