
Our promise to you:
Guaranteed product quality, expert customer support.
 24x7 CUSTOMER SERVICE
24x7 CUSTOMER SERVICE
 CONTACT US TO ORDER
CONTACT US TO ORDER
CRISPR-Cas9 Genome Editing for Treating Sickle Cell Disease 
Sickle cell disease (SCD) is a severe hereditary form of anemia, which is caused by a single mutation at the sixth codon of the β-globin chain of the adult hemoglobin (Hb) tetramer (α2β2). It is one of the most common and severe monogenetic disorders, and more than 100 000 individuals in the United States and several million around the world are affected by both acute and chronic manifestations of SCD, such as silent cerebral infarct, frequent pain crises, stroke, end organ damage and early death. Polymerized sickle hemoglobin (HbS, α2βS2) interferes with red blood cell biconcave architecture and flexibility, leading to crescent-shaped cells with enhanced adherence to the vascular endothelium, and hemolysis, which obstructs blood flow.
CRISPR-Cas9 Genome Editing in
SCD is considered as a therapeutic target for the gene editing ability of CRISPR/Cas9, as it is a monogenic recessive disorder, caused by a substitution in the β‐globin gene that alters one amino acid. Using established methodology, CRISPR/Cas9 has been used to edit genomes of human induced pluripotent stem cells (iPSCs) as a method to provide gene‐corrected cells for SCD. The results based on HBB protein expression are encouraging, suggesting that CRISPR/Cas9 genome editing of SCD iPSCs could be used for disease modeling and potential gene therapy. After reporting the curative strategy using CRISPR/Cas9‐ mediated HBB targeting for hematopoietic stem cells in SCD disease patients and other β‐ hemoglobinopathies, there is increased reliability of clinical trials using the method of ex vivo gene correction followed by autologous transplantation.
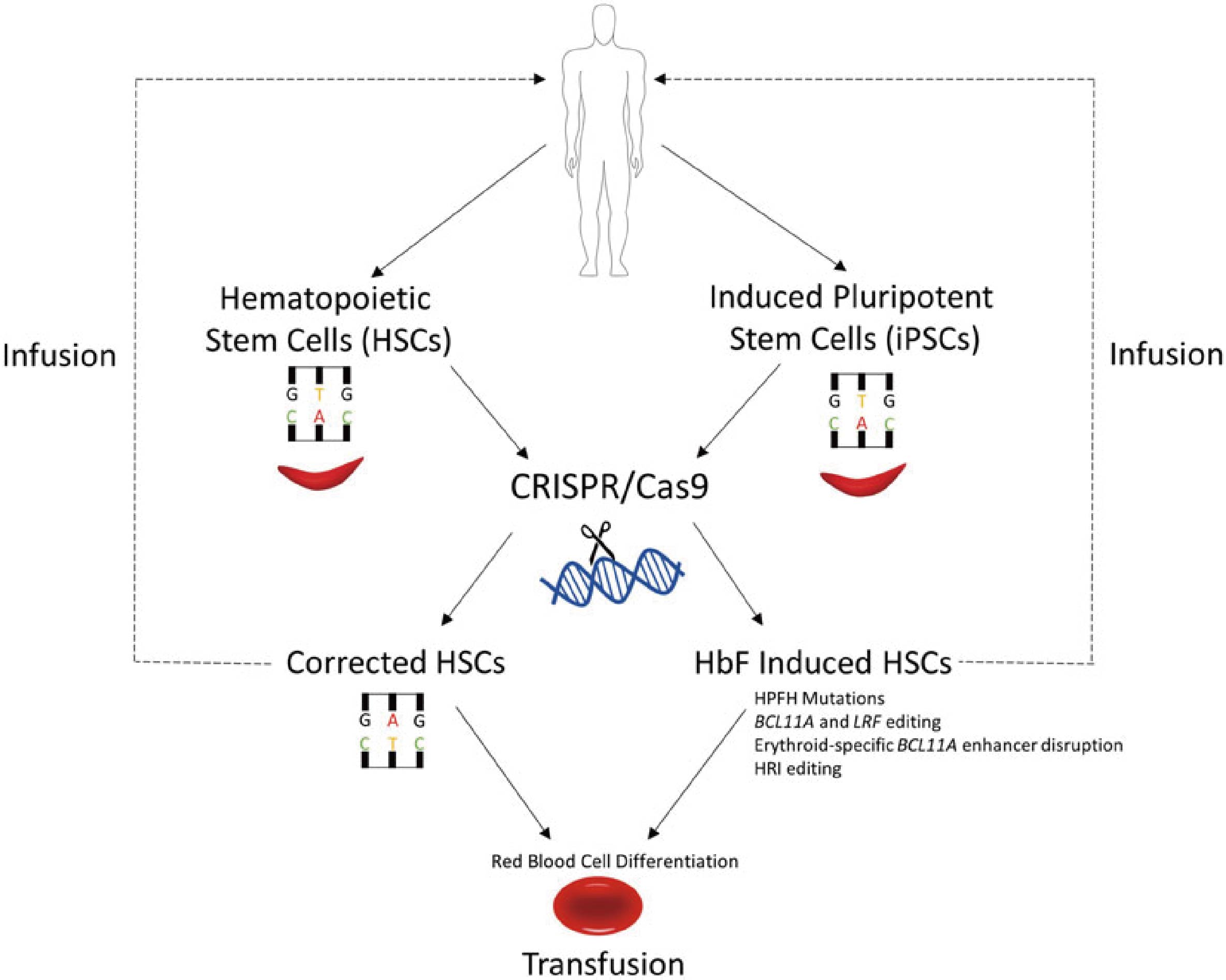 Figure 1. Potential CRISPR/Cas9 applications for sickle cell disease (SCD). (Demirci S, et al., 2019)
Figure 1. Potential CRISPR/Cas9 applications for sickle cell disease (SCD). (Demirci S, et al., 2019)
➢ Fetal hemoglobin (HbF) Induction
The initial observation by Janet Watson et al. shows that newborn babies will not have SCD complications for a certain period due to high levels of HbF in the infant's blood, and then more work has been devoted to increasing HbF levels in the adult body. The important role of elevated HbF for SCD protection has been further confirmed with the reports showing asymptomatic patients with SCD with elevated HbF due to the hereditary persistence of fetal globin (HPFH) mutations. Such mutations occur either in the form of large deletions in the β-globin gene, or smaller deletions/single nucleotide polymorphisms (SNPs) of quantitative trait loci (QTL) regulated by γ-globin promoter or HbF. Point mutations created by CRISPR/Cas9 method in the -115 and -200 clusters of the γ-globin promoter, inhibiting the binding of validated HbF transcriptional repressors BCL11A and LRF, respectively, de-repressed the expression of HbF. In a recent paper, protein kinase domain-focused CRISPR/Cas9-based genetic screening showed that heme-regulated inhibitor HRI, an erythroid specific kinase that controls protein translation as an HbF repressor, can be used as a potential candidate for the treatment of hemoglobinopathies.
➢ SCD Mutation Correction
Two studies have been done with CRISPR/Cas9 to show that biologically, SCD is curable. In one study, CRISPR/Cas9 has successfully edited out the sickle cell gene in human stem cells. The study also used stem cells from patients with β thalassemia. Work done has been sufficiently successful for researchers to start preparing for FDA approval of human trials. In another study, CRISPR/Cas9 edited human hematopoietic stem/progenitor human cells to reduce the amount of sickled hemoglobin RNA produced for over four months, suggesting that the treatment could have therapeutic benefits. Besides, reproducible protocols have proved how to achieve genetic mutations in human hematopoietic stem cell transplantation-based therapies like those for SCD. In other forms of anemia like Fanconi anemia, somatic cells taken from patients have successfully been reprogramed to produce patient-specific iPSCs.
Our CRISPR/Cas9 System Services
CRISPR/Cas9 PlatformCB is committed to providing the most professional and comprehensive genetic editing technology solutions for our clients. To support your projects, we offer a comprehensive custom CRISPR/Cas9 gene editing service from strategy design to final SCD model generation.
➢ Establishment of gene-edited cells
➢ Animal models generation of SCD by CRISPR/Cas9 system
If you have any questions, please feel free to contact us.
Related Services and Products
Animal Models
- Conditional Knockout Mouse
- Conventional Knockout Mouse
- Point Mutation Mouse
- CRISPR/Cas9 Knockin Mouse
- Rosa26 Knockin Mouse
Cell Lines
- Point Mutation Cell Line Generation
- HIEF™ Site-Specific Knock-in Cell Line Service
- Fragment Deleted Stable Cell Line
- Gene Editing in Primary T Cells
- Gene Knockout Cell Line Generation
Products
- CRISPR/Cas9 Kits
- Pre-made Knockout Cell Line
- CRISPR Lentiviral Library
- Cas9 Related Plasmids
- Pre-made Cas9 Virus Particles
References
- Baffoe-Bonnie M S. A justice-based argument for including sickle cell disease in CRISPR/Cas9 clinical research. Bioethics, 2019, 33(6): 661-668.
- Kapoor S, et al. Advances in the treatment of sickle cell disease//Mayo Clinic Proceedings. Elsevier, 2018, 93(12): 1810-1824.
- Demirci S, et al. CRISPR/Cas9 for sickle cell disease: applications, future possibilities, and challenges//Cell Biology and Translational Medicine, Volume 5. Springer, Cham, 2019: 37-52.
- Frangoul H, et al. CRISPR-Cas9 Gene Editing for Sickle Cell Disease and β-Thalassemia. New England Journal of Medicine, 2020.
- Demirci S, et al. Gene therapy for sickle cell disease: An update. Cytotherapy, 2018, 20(7): 899-910.
- Jayavaradhan R, Malik P. Genetic therapies for sickle cell disease. Pediatric Clinics, 2018, 65(3): 465-480.
- Tasan I, et al. Use of genome-editing tools to treat sickle cell disease. Human genetics, 2016, 135(9): 1011-1028.