
Our promise to you:
Guaranteed product quality, expert customer support.
 24x7 CUSTOMER SERVICE
24x7 CUSTOMER SERVICE
 CONTACT US TO ORDER
CONTACT US TO ORDER
CRISPR-Cas9 Genome Editing for Huntington's Disease 
Huntington's disease (HD) is an autosomal dominant neurodegenerative condition, which is caused by a pathogenic repeat expansion of the cytosine-adenine-guanine (CAG) trinucleotide in exon 1 of the huntingtin gene (HTT) on chromosome 4. This genetic change encodes an expanded polyglutamine tract near the amino terminus of the HTT protein. Healthy individuals have 17-35 repeats, while one of the two HTT alleles has a CAG expansion >35-40 repeats in HD patients. Longer repeat lengths have been shown to result in earlier onset disease of increased severity. Additional genetic modifier loci may also affect disease onset and progression. The clinical hallmarks of adult onset HD are abnormal movement, cognitive decline and behavioral or psychiatric changes.
Current Huntington's Disease Treatment Strategies
HD is an autosomal dominant disorder mainly because of the gain of functions, and thus a natural therapeutic target. The main focus has shifted to targeting the mutant HTT (mHTT) production pathway to address the most proximal cause of HD pathogenesis. These strategies can be RNA or DNA-based. HTT pre-mRNA can be targeted by using antisense oligonucleotides (ASOs) and mature mRNA with RNA interference (RNAi), both of which can promote early degradation of mHTT and reduce the level of mHTT. However, these methods induce partial degradation of the target mRNA and therefore hypomorphic phenotypes. Besides, they require long-term and continuous expression of the therapeutic molecules. On the other hand, HTT gene editing relies on a transient treatment leading to permanent inactivation/repair of the HTT gene. The mutated HTT gene can be targeted by using zinc finger motif proteins (ZFPs) that repress transcription or CRISPR-Cas9 that can edit DNA sequences. The design and implementation of a therapeutic construct that directly interacts with DNA to reduce the transcription of mutant HTT gene present both different challenges and greater reward potential than targeting of RNA. DNA-targeting therapeutic agents would be expected to improve all aspects of Huntington's disease, including those mediated by alternative splicing, non-RAN translation, or any other mechanism. In addition, DNA editing opens up the future prospect of germ-line treatment in living patient tissue, which could benefit future offspring in addition to those carrying mutations.
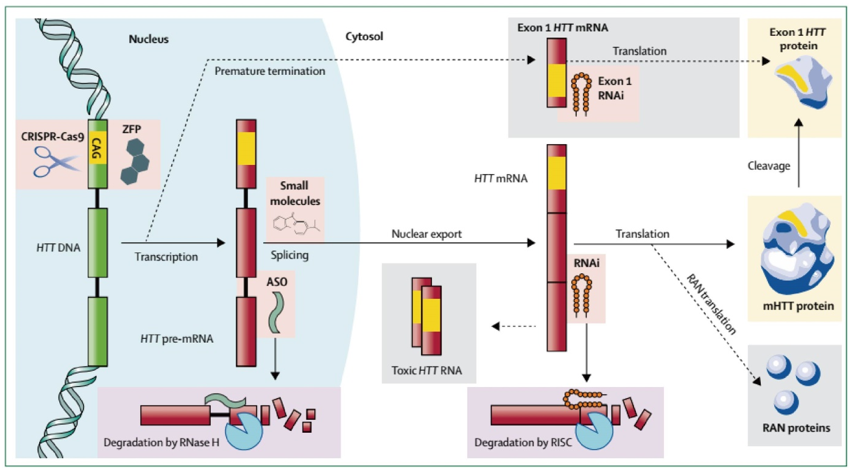 Figure 1. Production of HTT and targeted molecular therapies in development to reduce its expression. (Wild E J, Tabrizi S J. 2017)
Figure 1. Production of HTT and targeted molecular therapies in development to reduce its expression. (Wild E J, Tabrizi S J. 2017)
CRISPR-Cas9 System in Huntington's Disease Treatment
The CRISPR/Cas9 system is a gene-editing strategy, which involves the highly specific identification of a double-stranded DNA sequence through the CRISPR system followed by an RNA-guided nuclease (Cas9 protein) that leads to the breakage and excision of the DNA sequence. For gene replacement methods suitable for HD, there is a need for a protospacer adjacent motif sequence, which allows a specific recognition site in SNP alleles of the mutated HTT gene. Conceptually, the CRISPR/Cas9 system may be used to remove the mHTT gene and replace it with the wild type allele, suppress the mutated allele by inserting a missense mutation, or reduce transcription of the HTT gene in a non-allele specific manner, such as epigenetic regulation.
CRISPR/Cas9 system-based methods are at very early stages of therapeutic development, with a few proof-of-concept studies in cell cultures of HD patients suggesting the ability of the CRISPR/Cas9 system to excise the mutated allele and prevent the production of the mHTT protein. The method was first used to inactivate the mutant HTT allele permanently and selectively in patient-derived fibroblasts, with two constructs to excise a large region of HTT DNA, leading to near-total reduction in both RNA and mutant HTT protein. In 2017, the method was successfully tested in a rodent model of Huntington's disease, generating selective mutant HTT reduction, attenuation of pathology, and improved motor function, but not extended survival, after treatment of the striatum with a CRISPR-Cas9 construct that deleted the region containing the CAG repeat in the human HTT gene of Q140 knockin mice.
However, the application of CRISPR-Cas9 in Huntington's disease is still in the early stages. A lot of work is needed to bring these rapidly evolving technologies to the clinic.
Our CRISPR/Cas9 System Services
CRISPR/Cas9 PlatformCB is committed to providing the most professional and comprehensive genetic editing technology solutions for our clients. To support your projects, we offer a comprehensive custom CRISPR/Cas9 gene editing service from strategy design to final HD model generation.
➢ Human Cell Models Generation of HD by CRISPR/Cas9 System
➢ Animal Models Generation of HD by CRISPR/Cas9 System
If you have any questions, please feel free to contact us.
Related Services and Products
Animal Models
- Conditional Knockout Mouse
- Conventional Knockout Mouse
- Point Mutation Mouse
- CRISPR/Cas9 Knockin Mouse
- Rosa26 Knockin Mouse
Cell Lines
- Point Mutation Cell Line Generation
- HIEF™ Site-Specific Knock-in Cell Line Service
- Fragment Deleted Stable Cell Line
- Gene Editing in Primary T Cells
- Gene Knockout Cell Line Generation
Products
- CRISPR/Cas9 Kits
- Pre-made Knockout Cell Line
- CRISPR Lentiviral Library
- Cas9 Related Plasmids
- Pre-made Cas9 Virus Particles
References
- Vachey G, Déglon N. CRISPR/Cas9-mediated genome editing for Huntington's disease. Huntington's Disease. Humana Press, New York, NY, 2018: 463-481.
- Bashir H. Emerging therapies in Huntington's disease. Expert review of neurotherapeutics, 2019, 19(10): 983-995.
- Dash D, Mestre T A. Therapeutic update on Huntington's disease: symptomatic treatments and emerging disease-modifying therapies. Neurotherapeutics, 2020: 1-15.
- Wild E J, Tabrizi S J. Therapies targeting DNA and RNA in Huntington's disease. The Lancet Neurology, 2017, 16(10): 837-847.
- Kolli N, et al. CRISPR-Cas9 mediated gene-silencing of the mutant huntingtin gene in an in vitro model of Huntington's disease. International journal of molecular sciences, 2017, 18(4): 754.
- Yang S, et al. CRISPR/Cas9-mediated gene editing ameliorates neurotoxicity in mouse model of Huntington's disease. The Journal of clinical investigation, 2017, 127(7): 2719-2724.