
Our promise to you:
Guaranteed product quality, expert customer support.
 24x7 CUSTOMER SERVICE
24x7 CUSTOMER SERVICE
 CONTACT US TO ORDER
CONTACT US TO ORDER
Application of CRISPR/Cas9 Gene Editing Technique in HIV/AIDS Therapy 
Each year, more than two million people are newly infected with human immunodeficiency virus (HIV), thus acquired immunodeficiency syndrome (AIDS) remains a worldwide health problem. More than 20 years after its introduction, combination antiretroviral therapy (cART) is still the only available treatment for individuals infected with HIV-1. The current antiretroviral regimens are effective in suppressing viral replication and decreasing transmission rates and have significantly improved HIV individual's life expectancy. Unfortunately, cART interruption invariably results in the rebound of plasmatic viremia in treated individuals, to similar levels than before treatment initiation. Because the therapy is not curative, HIV individuals must adhere to a lifelong daily antiretroviral drug regimen, which has led to a new series of complications of sustained chronic inflammation, premature aging and higher risks of non-AIDS comorbidities.
Recent advances in the field of gene engineering demonstrate that it is a routine to modify the genomes by precisely targeted inserting, deleting, or replacing specific DNA sequences. So far, three main classes of targetable nucleases, including zinc finger nucleases (ZFNs), transcription activator‐like effector nucleases (TALENs), and RNA‐guided engineered nucleases (RGENs) that are derived from the clustered regularly interspaced short palindromic repeat (CRISPR)‐Cas system, are routinely used for this purpose. Besides, CRISPR/Cas9 evolved from type II CRISPR/Cas system has triggered innovative applications in life sciences, acting as a “sharp‐sword” for genome editing. The rise in the use of CRISPR/Cas9 system in treating HIV infection has aroused great interest among researchers.
CRISPR/Cas9 for Novel Gene Editing AIDS Therapies
The CRISPR/Cas9 system can be used to modify host cells in this way that they are no longer susceptible to HIV infection. Inspired by the successful cure of the 'Berlin patient', who underwent allogeneic stem cell transplantation with donor cells lacking the CCR5 coreceptor, several studies focused on targeting the CCR5 gene. However, CCR5 inactivation may trigger envelope mutations that shift viral receptor usage from CCR5 to CXCR4. CRISPR/Cas9 system can also target CXCR4 or other cellular factors involved in HIV replication, however, this may have undesirable side effects on cell physiology. Apart from targeting host co-factors, the viral DNA can be targeted directly by the introduction of the Cas9 protein and anti-viral gRNA in HIV-infected cells. Otherwise, cells can be harnessed with the CRISPR reagents to immediately attack and cleave the reverse-transcribed viral DNA that is produced upon infection. In reality, the reported inhibition of virus replication in Cas9/gRNA harnessed cells is probably caused by the combined effects on HIV DNA before and after integration.
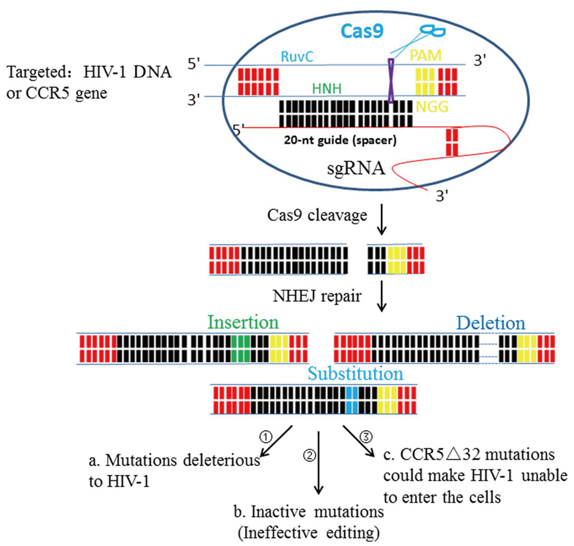 Figure 1. HIV‐1 DNA or CCR5 gene editing by using CRISPR/Cas9 system. (Deng Q, et al. 2018)
Figure 1. HIV‐1 DNA or CCR5 gene editing by using CRISPR/Cas9 system. (Deng Q, et al. 2018)
The CRISPR/Cas9 technology can disrupt both cellular genes necessary for HIV-1 infection and integrated HIV-1 proviral DNA. It has been indicated that CRISPR/Cas9 facilitates the excision of DNA segments of integrated HIV-1 provirus DNA in a variety of latently infected cell types, including CD4+ T cells. CRISPR/Cas9 system can be targeted to sequences within the HIV-1 LTR U3 region that flanks the proviral genome and thus allows the complete excision of the proviral DNA. This method also allows cells to be prophylactically protected by expressing CRISPR/Cas9 in uninfected cells to prevent them from being infected later by HIV-1. However, this approach requires longer, or more constitutive, expression of CRISPR/Cas9 in the cells and the issue of the immunogenicity of Cas9 has not yet been resolved. In a recent study, tail-vein or intraperitoneal injection of two transgenic mouse models with recombinant adeno-associated virus 9 vector expressing Cas9 and a multiplex of gRNAs affected the cleavage of integrated HIV-1 DNA in the animal. Resection of a large essential DNA fragment from the HIV-1 provirus occurred in the heart, spleen, lung, liver, kidney, and circulating lymphocytes of the mice indicating proof-of-concept experiments for the in vivo eradication of integrated HIV-1 provirus by CRISPR/Cas9 in a variety of different cells and tissues.
CRISPR/Cas9 System Delivery Approach
The major challenge of curing HIV/AIDS is the persistence of multiple reservoirs in several drug-inaccessible parts of the human body. Besides, while current HIV/AIDS patients can take medications to suppress symptoms, the side effects of these drugs may lead to high morbidity in these patients. Since its discovery, the CRISPR/Cas9 system has been a leading tool in gene editing. Currently, the delivery approaches of CRISPR/Cas9 system are divided into viral and non-viral delivery. The CRISPR/Cas9 system is very flexible and can be delivered in the form of DNA, RNA, or RNA/protein complex. The most popular method is the DNA form as one or several plasmids. In the viral delivery methods, lentivirus, AAV, and baculovirus have been tried. No matter which delivery strategy is being used, the emphases are biocompatible and targetable carriers, low-invasive administration, controllable reagents release. As for HIV/AIDS eradication, in order to establish more efficient evaluation methods, HIV/AIDS primate models will be the center of the next phase of the study. Future efforts will be dedicated to the efficient delivery of CRISPR/Cas9 system components to latently infected cells.
Related Services and Products
Animal Models
- Conditional Knockout Mouse
- Conventional Knockout Mouse
- Point Mutation Mouse
- CRISPR/Cas9 Knockin Mouse
- Rosa26 Knockin Mouse
Cell Lines
- Point Mutation Cell Line Generation
- HIEF™ Site-Specific Knock-in Cell Line Service
- Fragment Deleted Stable Cell Line
- Gene Editing in Primary T Cells
- Gene Knockout Cell Line Generation
Products
- CRISPR/Cas9 Kits
- Pre-made Knockout Cell Line
- CRISPR Lentiviral Library
- Cas9 Related Plasmids
- Pre-made Cas9 Virus Particles
References
- Verdikt R, et al. Applications of CRISPR/Cas9 tools in deciphering the mechanisms of HIV-1 persistence. Current opinion in virology, 2019, 38: 63-69.
- Huang Z, et al. Current application of CRISPR/Cas9 gene-editing technique to eradication of HIV/AIDS. Gene therapy, 2017, 24(7): 377-384.
- Deng Q, et al. Developmental progress of CRISPR/Cas9 and its therapeutic applications for HIV‐1 infection. Reviews in medical virology, 2018, 28(5): e1998.
- Das A T, et al. Elimination of infectious HIV DNA by CRISPR–Cas9. Current opinion in virology, 2019, 38: 81-88.
- Khalili K, et al. Genome editing strategies: potential tools for eradicating HIV-1/AIDS. Journal of neurovirology, 2015, 21(3): 310-321.
- Khalili K, et al. Novel AIDS therapies based on gene editing. Cellular and Molecular Life Sciences, 2017, 74(13): 2439-2450.