An international team led by KAUST found an epigenetic mechanism for regulating gene activity in the study of the adult genomes and their interactions with environment.
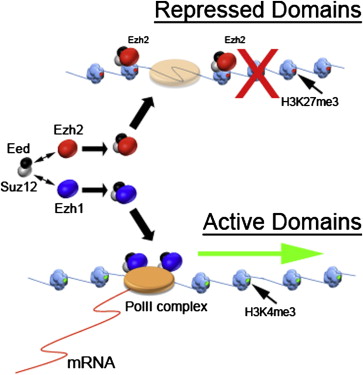
The Valerio Orlando laboratory from KAUST has been studying the role of Ezh1, while the functional study of this gene in mature tissue has not progressed in the past 25 years. Like its homologous gene—Ezh2, Ezh1 and its chaperone protein together encode a protein that binds the target gene to inhibit its activity. However, although the Ezh2 mutation is associated with cancer and developmental defects, mice carrying Ezh1 mutations appear to develop normally.
Seven years ago, Professor Orlando's team found that Ezh1 could be combined with many of the gene promoters that could normally initiate gene transcription. "We found these typical epigenetic inhibitors bind to the active genes, so we speculate that they can inhibit the expression of these genes," Orlando said. They suspected that this inhibitory effect may be triggered by stress, so they gave pressure on the muscle cells and found that only in the Ezh1-expressing cells can be observed the inhibition. The stress caused Ezh1 activation, thereby giving the gene a repressive label that can be cleared. Professor Orlando called this process as "cell plasticity": the ability of the cell to adapt to the dynamic environment.
A turning point in the understanding of Ezh1 occurred when a shortened version of the protein was discovered. Many genes in the body will encode several structures of a protein which are less different and are called isotype proteins. So the team realized that this shorter Ezh1 isotype protein might have some other functions.
"When we turn our attention to this short Ezh1, we immediately find some phenomena," Orlando recalled. This shortened Ezh1 was present in the cytoplasm rather than nucleus. The research team found that it played the function of the environmental sensor and could regulate the activity of integral Ezh1. In order to label the target gene, Ezh1 required a chaperone protein, and the short Ezh1 can bind to its partner and transfer it to cytoplasm, just like "tethering the protein with a rope." When the cells were under pressure, the short Ezh1 degraded and released chaperone protein to bind with the integral Ezh1 in the nucleus. Once the pressure was stopped, the short Ezh1 can once again bind the chaperone protein, inhibiting the activity of integral Ezh1 and clearing the inhibitory label tagged on particular gene.
This finding reveals a novel epigenetic regulatory mechanism because interactions occur between different proteins expressed by the same gene, rather than between different gene expression products. "This provides a new model for gene regulation that associates the genome with the environment," Orlando said. "It's a very exciting new discovery."
Related links: