• Adenovirus Service • AAV Service • Lentivirus Service • Retrovirus Service



Most cancers exhibit aneuploidy, but its functional significance in tumorigenesis is controversial. Recently, in a research report titled "Oncogene-like addiction to aneuploidy in human cancers" published in the international journal Science, scientists from Johns Hopkins University School of Medicine and other institutions found that cancer cells with extra chromosomes may rely on these chromosomes to fuel tumor growth. Eliminating these extra chromosomes prevents the cells from forming tumors. Related research results suggest that selectively targeting extra chromosomes may provide a new way to treat cancer.
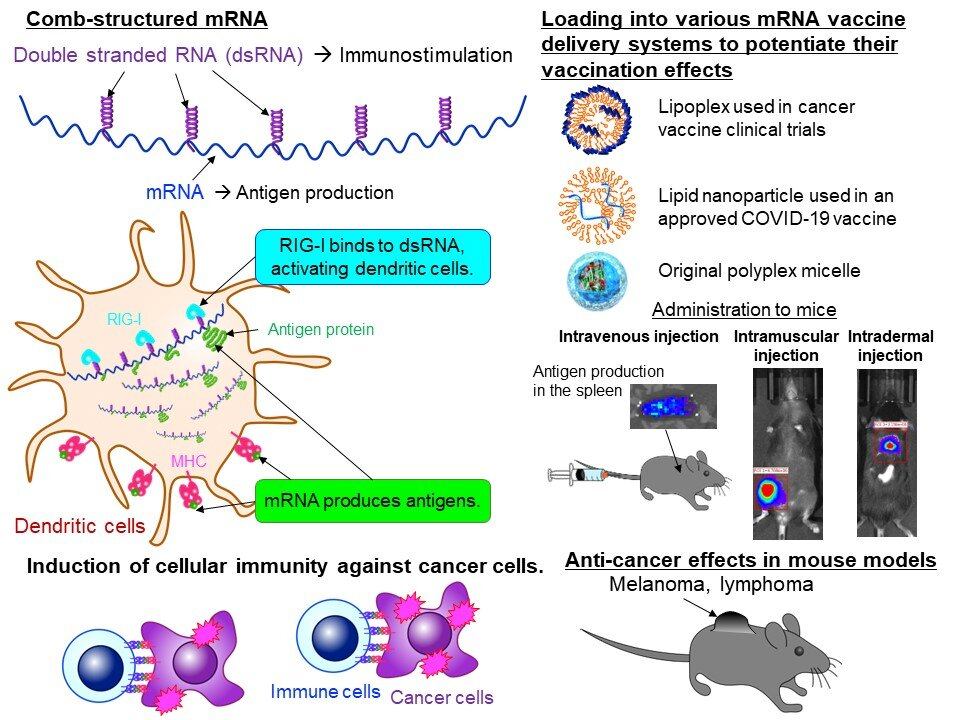
The mRNA COVID-19 vaccine has allowed us to witness the effectiveness, safety and great potential of mRNA technology. Currently, research institutions around the world are targeting cancer as the next target for mRNA technology. mRNA cancer vaccines activate cellular immunity to attack cancer cells by producing proteins (i.e., tumor antigens) specifically expressed in cancer cells. However, cancer cells are not easily distinguishable from normal cells, and cancer cells are immunosuppressive, making the development of mRNA cancer vaccines more challenging than mRNA infectious disease vaccines. Therefore, it is necessary to improve the efficacy of mRNA cancer vaccines, and enhancing immune activation through adjuvants is an effective strategy. But if the adjuvant is too strong, it will cause adverse reactions. An adjuvant that is too weak does not provide sufficient activation. Previous mRNA vaccines all have adjuvant effects, but there is a lack of rational and practical methods to obtain controllable adjuvant activity. Researchers from the Kawasaki Institute of Industrial Promotion in Japan published a research paper titled "Comb-structured mRNA vaccine tethered with short double-stranded RNA adjuvants maximizes cellular immunity for cancer treatment" in the Proceedings of the National Academy of Sciences. The study constructed a brand-new mRNA structural form, combining the single-stranded mRNA sequence encoding the antigen with the double-stranded RNA (dsRNA) to form a comb-like structure, in which the single-stranded mRNA is responsible for producing the antigen, and the dsRNA acts as an assistant. It can further activate immune cells, and has shown high anti-tumor effects in melanoma and lymphoma model mouse experiments. The intensity of immune stimulation can also be controlled by adjusting the amount of dsRNA, so as to achieve controllable adjuvant activity and ensure safety while improving vaccine efficacy. In this study, the research team used mRNA engineering techniques to develop a method to directly incorporate an adjuvant into single-stranded mRNA encoding an antigen without interfering with its ability to produce the antigenic protein. The research team designed a short dsRNA targeting the natural immune receptor RIG-I, and loaded it onto the single strand of mRNA by hybridization, thereby obtaining a comb-structured mRNA. By changing the dsRNA length and sequence, are able to efficiently activate RIG-1. Comb-structured mRNA generated by this method efficiently activates dendritic cells (DCs), which play an important role in vaccine efficacy. In addition, the intensity of immune stimulation can also be controlled by changing the amount of dsRNA bound to the single strand of mRNA. This is critical to achieve adequate vaccine efficacy while preventing excessive immune activation and ensuring safety. Next, the research team evaluated the effect of this new mRNA cancer vaccine in tumor mouse models. The results showed that when comb-structured mRNAs were loaded onto lipid nanoparticles (LNPs) used in clinical trials of cancer vaccines, the cellular immune activity necessary to attack cancer cells was significantly enhanced. In mouse models of melanoma and lymphoma, the tumors were significantly reduced in size and their lifespan was significantly extended.

Lung cancer is still the most common and deadly cancer in the world. Small cell lung cancer (SCLC) is the deadliest type of lung cancer, accounting for about 15% of all lung cancer cases. SCLC is an aggressive high-grade neuroendocrine tumor characterized by short doubling time, rapid growth, and early metastasis and spread. Most SCLC patients develop resistance rapidly, and their 5-year survival rate is very low (5-6%), even when the initial response to standard chemotherapy is good. The addition of immune checkpoint inhibitors to conventional chemotherapy for small cell lung cancer is promising; however, their absolute long-term benefits are moderate. The complex mechanisms of widespread SCLC metastasis and recurrence need to be clarified to expand the long-lasting benefits of chemotherapy and immunotherapy to more patients.
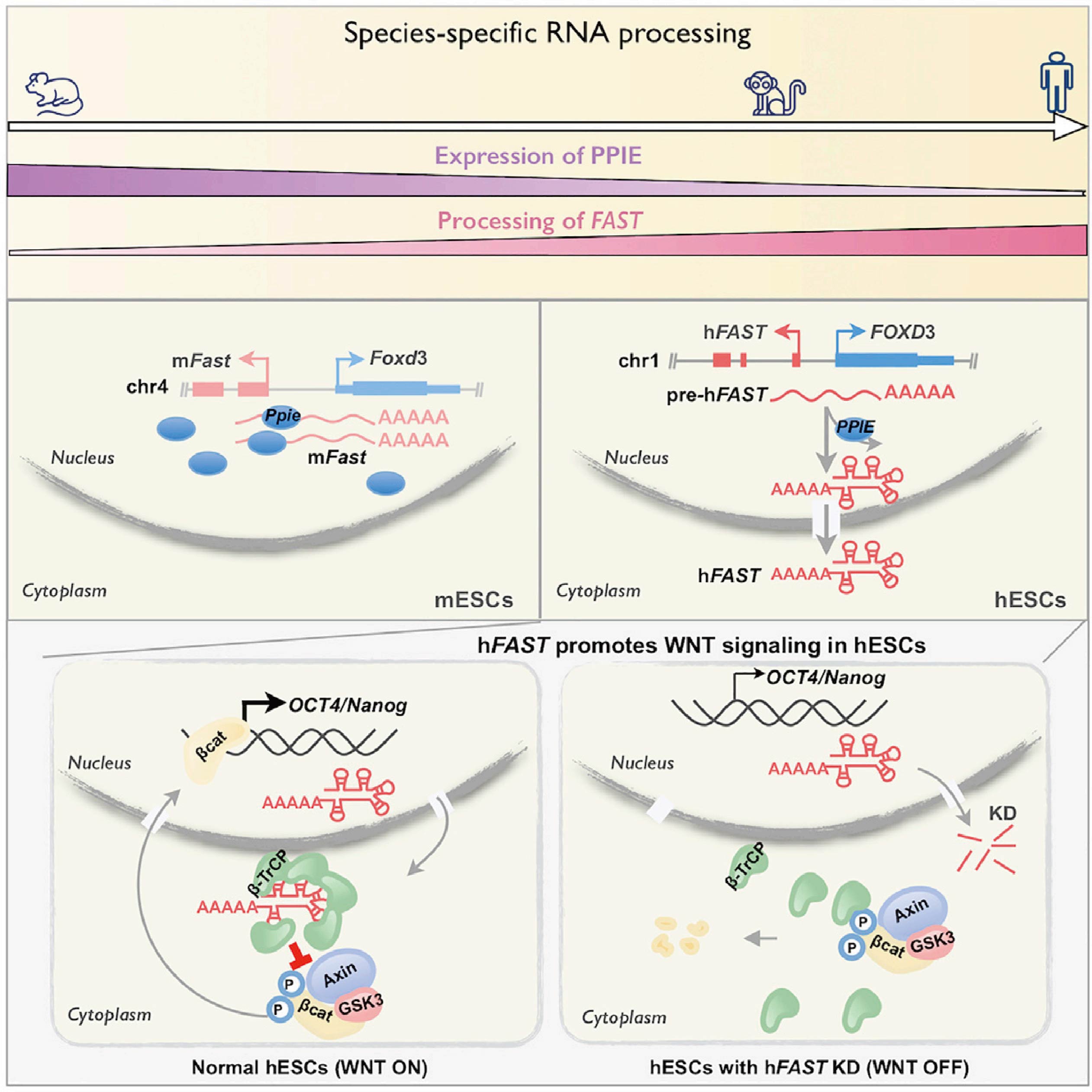
Long noncoding RNAs (lncRNAs) contain more than 200 nucleotides, lack protein-coding potential, and are widely transcribed in eukaryotic cell genes. At present, studies have shown that lncRNAs play a key role in gene expression in various cells and biological processes. Unlike conserved mRNAs, lncRNAs lack high sequence or secondary structure conservation. lncRNA conservation can also occur at the position and mechanism-of-action levels. Transcription of positionally conserved lncRNAs with nearby conserved coding genes among different species has been recognized as an indicator of potential functional significance. However, whether lncRNA processing is conserved and how processing contributes to its compartmentalization and function in different species remain unexplored.
New cancer immunotherapy involves extracting and genetically modifying patients' T cells so they can identify and attack tumors. This technology is a true medical breakthrough. Since CAR-T cell therapy was approved by the U.S. Food and Drug Administration (FDA) in 2017, more and more patients with leukemia and lymphoma have experienced complete remission. However, genetically modifying patients' T cells is laborious and expensive. Even if the treatment is successful, changes in the immune system can cause the patient to become seriously ill in a short period, with fever, nausea, and neurological symptoms.
In the last post, we have introduced some of the research achievements in the RNA molecular biology research, here are another achievements that are related to RNA molecular research as well:
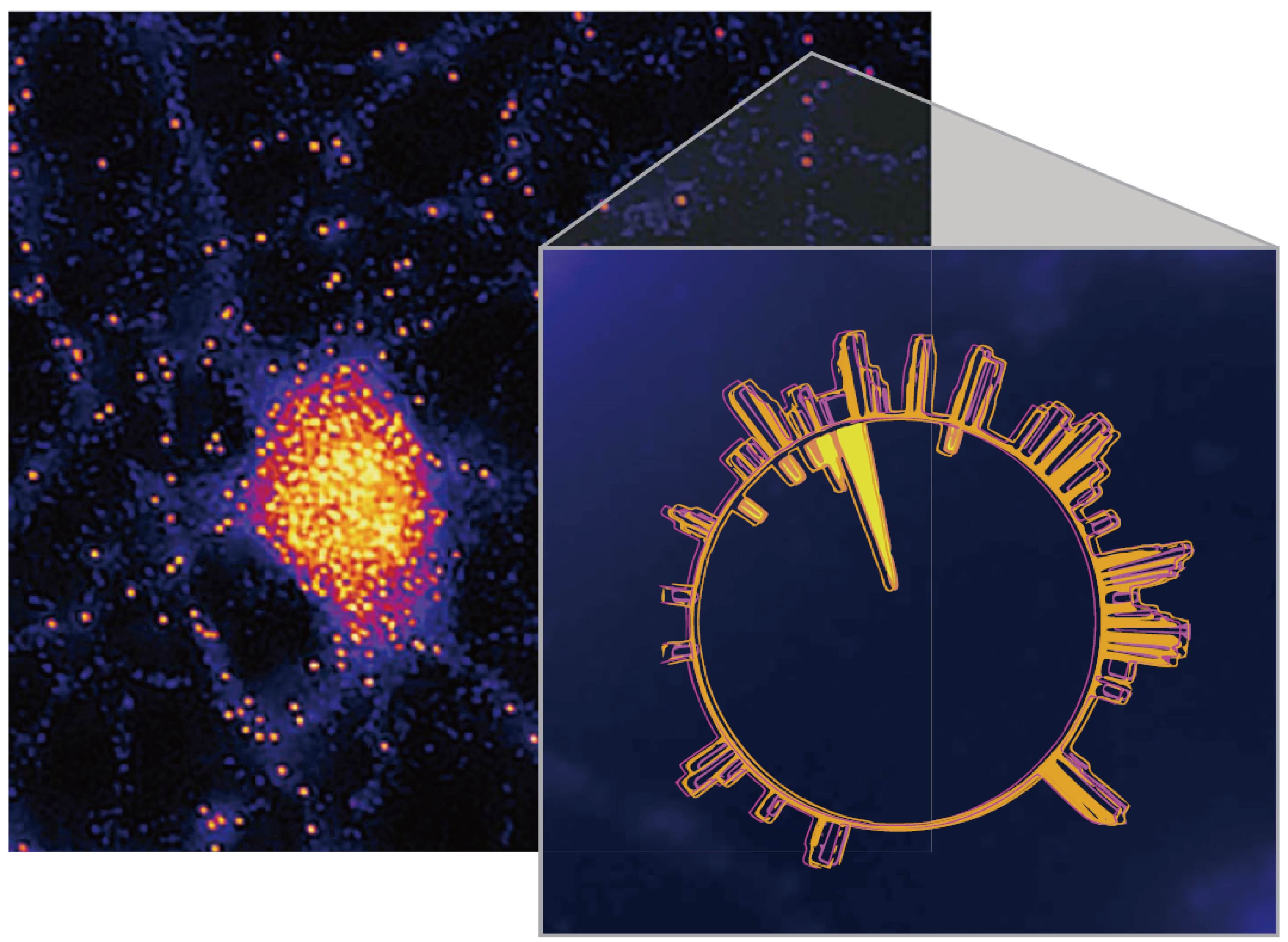
The Lasker Award, which is regarded as the ‘Wind Vane’ of the Nobel Prize. Professor Joan Argetsinger Steitz from Yale University won the 2018 Lasker Award for his 40 years of leadership in the field of bio-medicine, especially in the field of RNA biology. In this post, we will summarize the recent achievements in the field of RNA molecular biology research.
Life comes from a single cell (that is oosphere[oosperm]) that repeatedly divides to produce two cells, then four cells, then eight cells, up to about 26 billion cells that make up the newborn. A major challenge in developmental biology is to track how and when these 26 billion cells are produced from a fertilized egg. Until now, there are only snapshots for this developmental process being captured and analyzed in this field.
Hundreds of millions of years ago, plant and animal genomes were littered with viral DNA debris. So far, it has generally been assumed that most of the virus residues integrated into the plant and animal genomes are inactive. But in January 11th, two Cell articles confirmed that some of them might have evolved into the genes that promoted cellular communication by Drosophila and mice experiments.
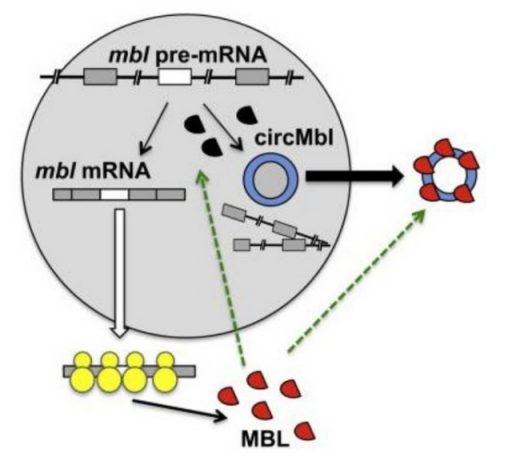
miRNA sponge functional model plays a very significant role in biological researches. In this blog, we mainly introduce professor Rajewsky Nikolaus, who have contributed a lot for the circular RNA function study.