• Adenovirus Service • AAV Service • Lentivirus Service • Retrovirus Service


What is mycoplasma?
Low transfection efficiency
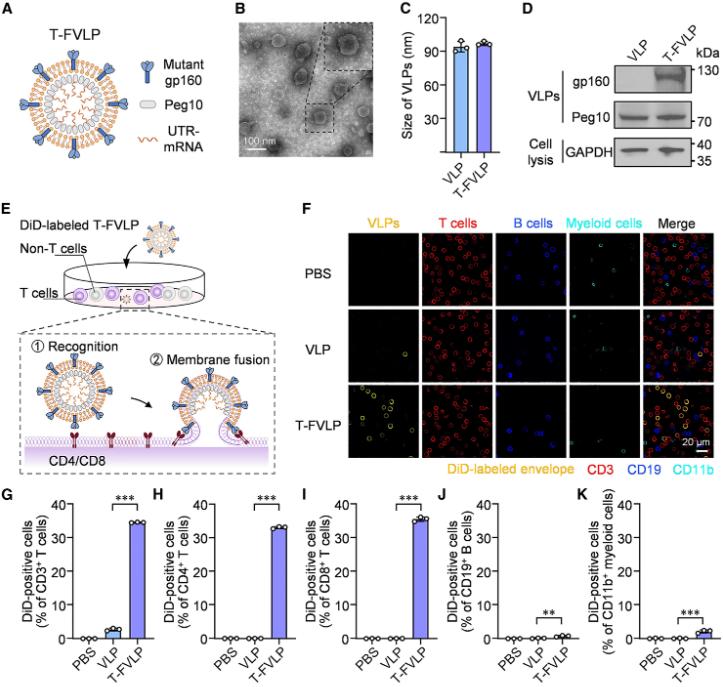
Researchers from South China University of Technology published a research paper titled "Leveraging T cell-specific fusogenicity of HIV for in vivo mRNA delivery to produce human CAR-T cells" in Cell Biomaterials, a subsidiary of Cell. The study used the T cell-specific fusion of HIV virus to develop a T cell-specific fusion virus-like particle (T-FVLP) that can mimic HIV virus and efficiently deliver CAR mRNA into T cells, thereby producing human CAR-T cells in vivo.
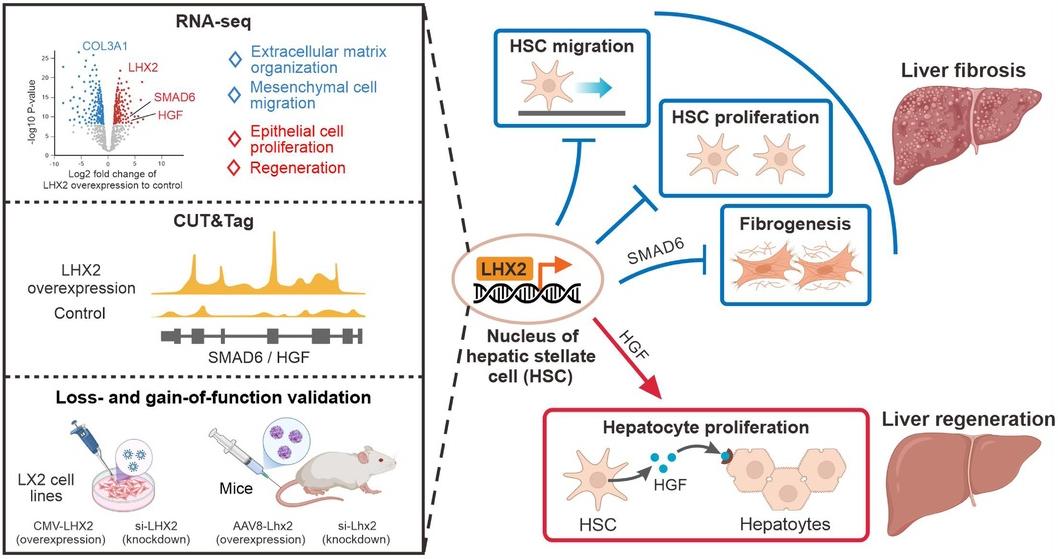
Promoting liver regeneration and inhibiting fibrosis is an attractive strategy for treating human liver diseases, and hepatic stellate cells (HSCs) are very important for both processes. Recently, in a research report entitled "Lhx2 specifically expressed in HSCs promotes liver regeneration and inhibits liver fibrosis" published in the international journal Hepatology, scientists from the Chinese Academy of Sciences identified the transcription factor Lhx2 (LIM homeobox protein 2) as a key regulator of hepatic stellate cells (HSCs). Lhx2 can simultaneously promote liver regeneration and inhibit liver fibrosis.

Recently, the latest research results published by researchers from the Saint Louis University School of Medicine in the journal Science Translational Medicine showed that cancer cells can produce tumor-derived extracellular vesicles (tEVs) containing PD-L1, causing PD-L1 to activate CREB and STAT signals, leading to lipid metabolism reprogramming of T cells. This induces T cell senescence and achieves immunosuppression. Blocking this process is expected to improve the sensitivity of solid tumors to immunotherapy such as PD-L1 inhibitors.
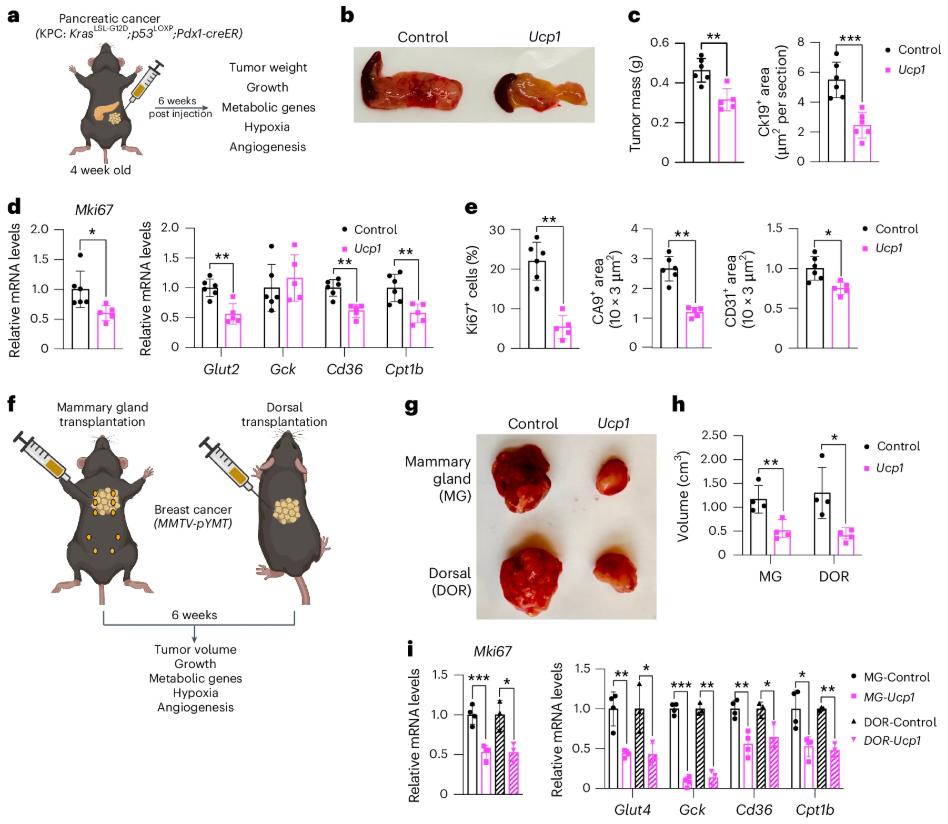
In the field of cancer research, the unique metabolic mode of tumor cells has always been the focus of scientists. In order to meet the needs of their own rapid proliferation, tumor cells will try their best to absorb and metabolize a large amount of nutrients. They can reprogram metabolic pathways and prefer to obtain energy through aerobic glycolysis even when there is sufficient oxygen. This phenomenon is called the "Warburg effect". At the same time, in an oxygen-deficient environment, tumor cells will increase the use of lipids.
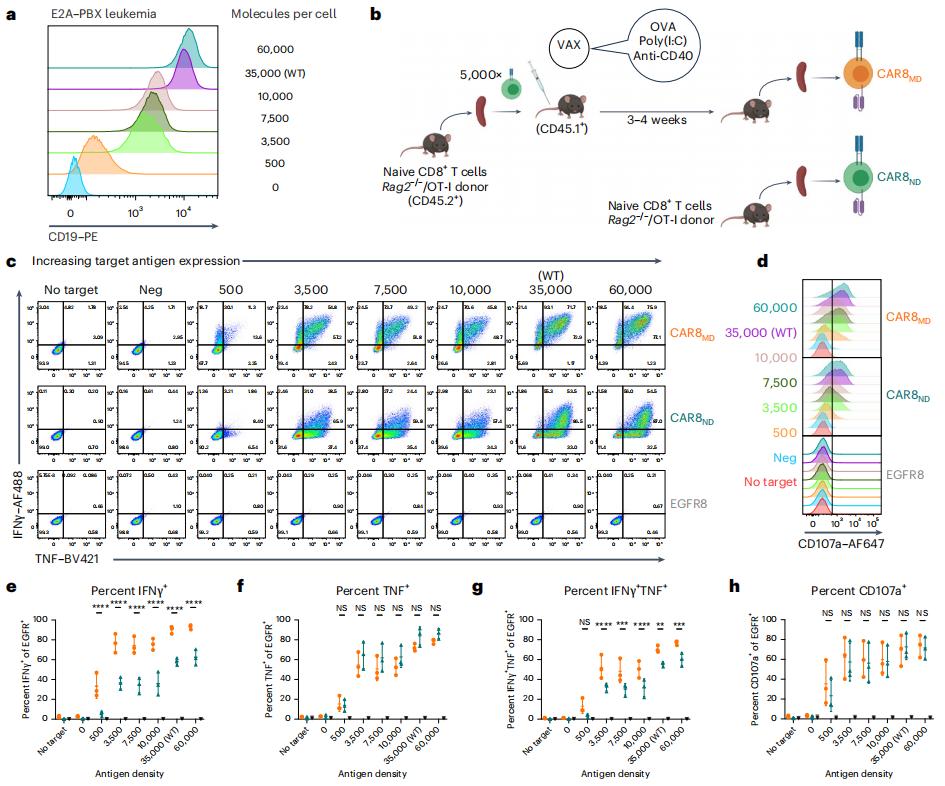
Although chimeric antigen receptor (CAR) T cells can effectively fight B-lineage malignancies, disease relapse is common in patients after CAR, and the therapeutic efficacy for other tumors is very limited. These challenges may be solved by controlling the function of CAR-T cells through reasonable manipulation.
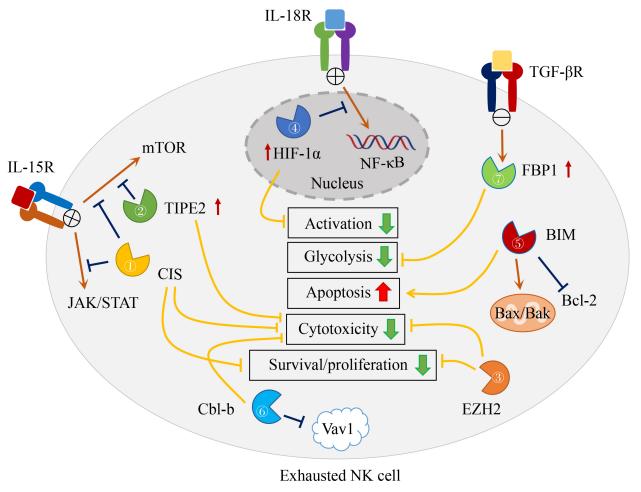
Recently, a review article discussing the therapeutic potential of natural killer (NK) cell biology was published in the journal Frontiers of Medicine. The article is titled "Intracellular checkpoints for NK cell cancer immunotherapy".
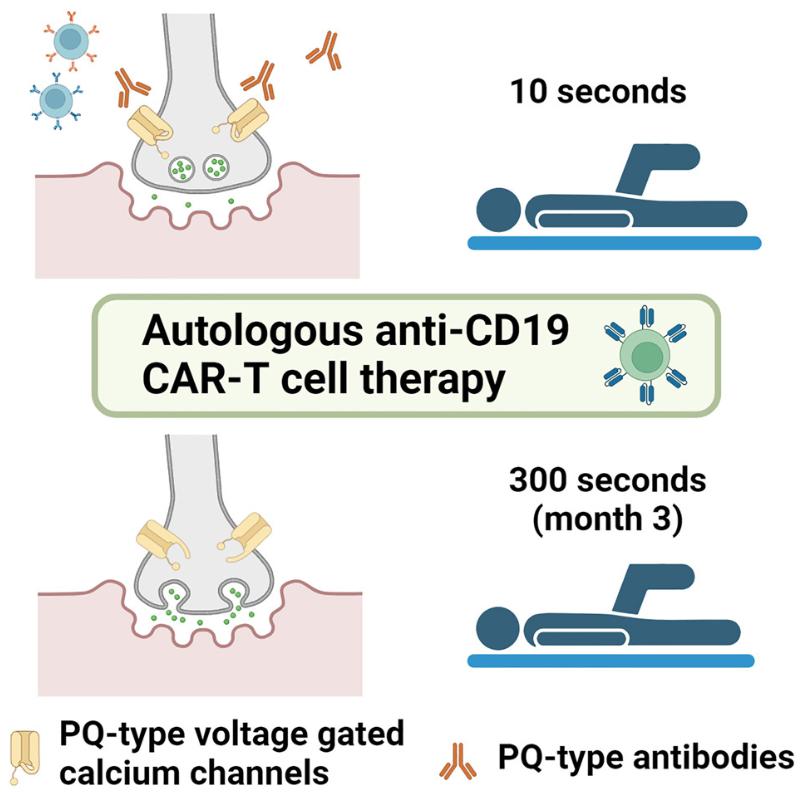
Lambert-Eaton myasthenic syndrome (LEMS), also known as myasthenic syndrome, is a rare, often tumor-associated autoimmune disease involving the presynaptic membrane of the neuromuscular junction that was first described in 1956. Typical clinical symptoms include progressive truncal muscle weakness, ptosis, as well as diplopia, dysarthria, and dysphagia. The disease is caused by pathogenic autoantibodies that target and inhibit the P/Q-type pressure-gated calcium channels (VGCCs) presynaptic to nerve terminals, which is different from the postsynaptic targets of pathogenic autoantibodies that cause myasthenia gravis (MG).
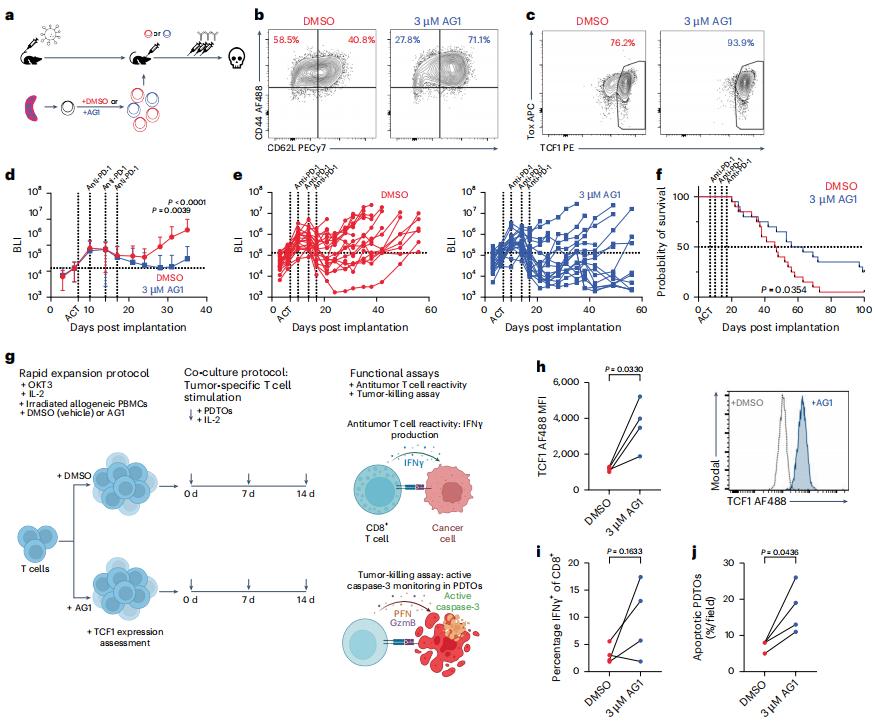
TCF1high progenitor CD8+ T cells can mediate the efficacy of immunotherapy, but researchers are currently unclear about the molecular mechanisms behind their generation and maintenance. Recently, in a research report published in the international journal Nature Immunology, entitled "Deficiency of metabolic regulator PKM2 activates the pentose phosphate pathway and generates TCF1+ progenitor CD8+ T cells to improve immunotherapy", scientists from Weill Cornell Medical College and other institutions found through a preclinical study that stimulating key metabolic pathways in T cells may enable them to more effectively resist and fight tumors when combined with immune checkpoint inhibitor therapy. This research finding may help researchers develop new potential strategies to enhance the potential of anti-cancer immunotherapy.