• Adenovirus Service • AAV Service • Lentivirus Service • Retrovirus Service



Breast cancer is one of the most common malignancies in women worldwide, with approximately 70% of cases being estrogen receptor-positive (ER+) subtypes. In recent years, CDK4/6 inhibitors (such as palbociclib) combined with endocrine therapy (such as tamoxifen) have become first-line treatment options for ER+ breast cancer, significantly prolonging progression-free survival. However, with widespread clinical application, drug resistance has become increasingly prominent, with approximately 30%-50% of patients experiencing disease progression after 2-3 years of treatment. The mechanisms of drug resistance are complex and diverse, including Rb protein loss and Cyclin E1 amplification, but the causes of resistance in a large number of cases remain unclear.

The median survival of patients with advanced pancreatic ductal adenocarcinoma (PDAC) is less than one year, highlighting the urgent need for treatment advances. Recently, a research paper titled "Clinical and molecular dissection of CAR T cell resistance in pancreatic cancer" was published in Cell Reports Medicine, a subsidiary of Cell. The paper reported a phase 1 clinical trial evaluating the safety and efficacy of anti-MSLN CAR-T cell therapy in patients with advanced pancreatic ductal adenocarcinoma (PDAC). Results showed that the CAR-T cell therapy was well tolerated but not highly effective. The research team further demonstrated in mouse studies that simultaneous knockout of both ID3 and SOX4 in these CAR-T cells improved long-term relapse-free survival.

Recently, a research team led by Aaron M. Ring of the Fred Hutchinson Cancer Research Center, Harriet M. Kluger of the Yale University School of Medicine, and Leon Furchtgott of the biotechnology company Seranova Bio published a major study in the top journal Nature.

CAR-T cell therapy has revolutionized the treatment of B-cell malignancies and is now showing initial success in solid tumors. Despite continued progress, many patients treated with CAR therapy fail to respond or develop resistance. This highlights the urgent need to further optimize current treatment options.

T-cell acute lymphoblastic leukemia (T-ALL) is a highly aggressive hematological malignancy, accounting for about 15% of childhood acute lymphoblastic leukemia (ALL) and 25% of adult ALL. Although the cure rate for pediatric patients can reach 80%, the long-term survival rate of adult patients is still less than 40%. More worryingly, more than half of patients will relapse after treatment or fail to respond to standard treatment. The median overall survival of relapsed/refractory T-ALL is only about 8 months. Current treatment methods mainly rely on high-intensity chemotherapy and allogeneic hematopoietic stem cell transplantation (alloHSCT), but they are highly toxic and have a high failure rate. There is an urgent need for safer and more effective targeted treatment strategies in clinical practice.

Glioblastoma (GBM) is the most common and aggressive type of malignant brain tumor in adults, with patients typically surviving only 12-18 months after diagnosis. Despite decades of research, there is currently no cure for glioblastoma, and approved treatments such as surgery, radiotherapy, and chemotherapy have limited effectiveness in extending patients' lives.

Data from the World Health Organization show that of the approximately 19.3 million new cancer cases worldwide each year, 10 million people die from cancer. Although immune checkpoint inhibitors (such as PD-1 antibodies) have revolutionized cancer treatment, a large number of patients still face the problem of "no response" or "drug resistance". Among them, natural killer cells (NK cells) are the first line of defense against cancer, and their activity is often inhibited by the tumor microenvironment. IL-15, as a core cytokine that regulates NK cell function, can enhance the killing ability of NK cells, but its clinical application is limited by the toxicity of systemic administration (such as cytokine storm).

Recently, a research team led by Jean-Christophe Marine and Joanna Pozniak of the Flemish Institute of Biotechnology (VIB) in Belgium, and Oliver Bechter of the University Hospital of Leuven, published an important research paper in the famous journal Cancer Discovery. When analyzing tumor samples from melanoma patients who did not respond to immunotherapy, they found that the number of natural killer (NK) cells increased significantly, and both NK cells and killer T cells gathered around the tumor. The amazing thing is that if the NK cells around the tumor are eliminated and then supplemented with immune checkpoint inhibitors, the killer T cells will infiltrate and destroy the tumor.
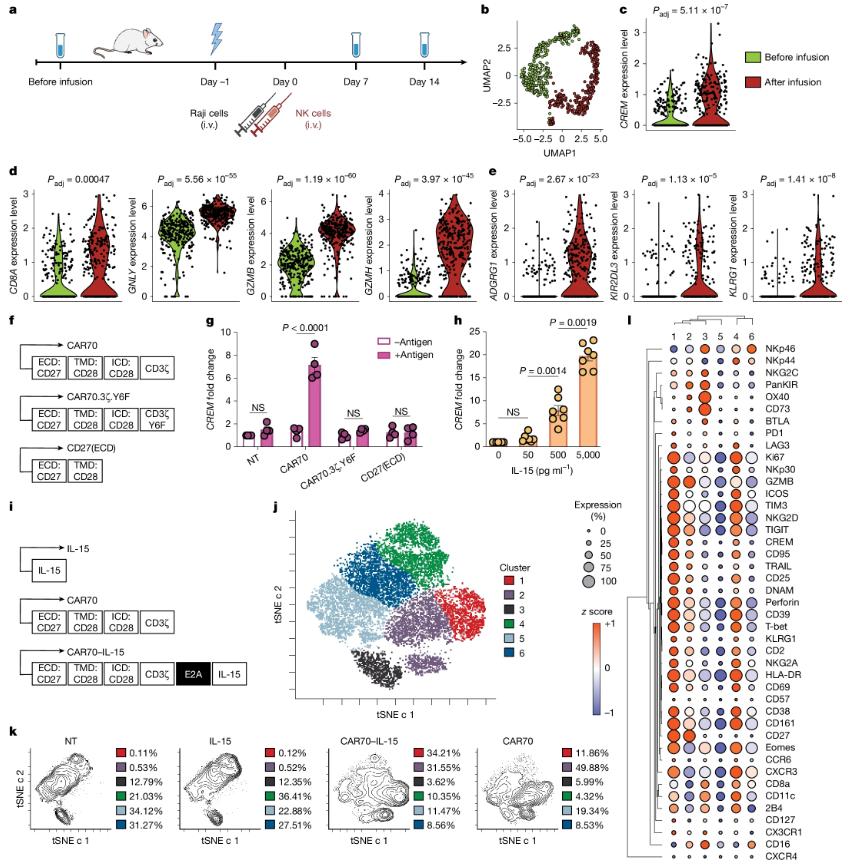
In the frontier field of cancer treatment, immunotherapy is gradually becoming a new hope for conquering cancer. In recent years, CAR-T cell therapy has achieved remarkable results in the treatment of blood tumors, but its application in the treatment of solid tumors still faces many challenges. At the same time, natural killer cells (NK cells), as an immune cell with strong anti-tumor activity, have gradually attracted the attention of researchers.
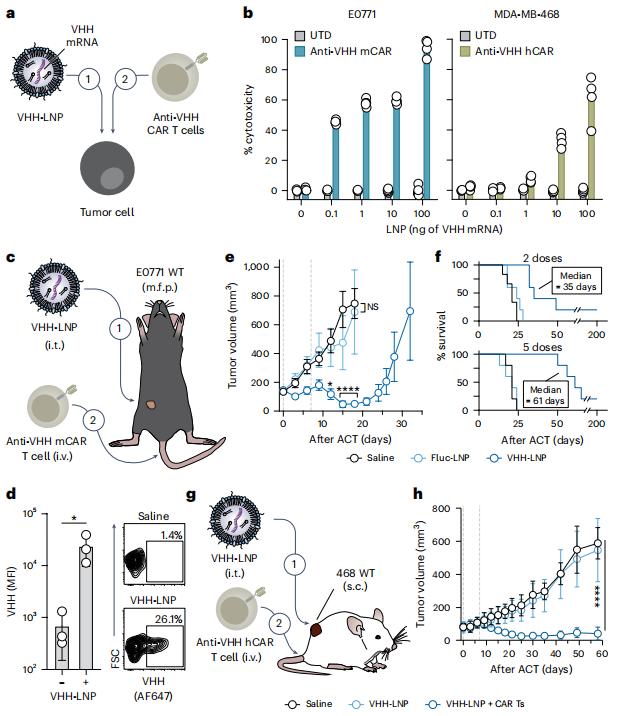
In a new study, researchers from the Georgia Institute of Technology have created a two-pronged approach that marks tumor cells so they can be recognized and destroyed by specially enhanced T cells from the patient's immune system. This approach could one day become a universal strategy for treating some of the most difficult-to-treat cancers, such as brain, breast and colon cancers, by teaching the immune system to find cancers it would normally miss. Their approach worked against these cancers in laboratory tests and did not harm healthy tissue. Importantly, it also prevented the cancer from coming back.