Our promise to you:
Guaranteed product quality, expert customer support.
 24x7 CUSTOMER SERVICE
24x7 CUSTOMER SERVICE
 CONTACT US TO ORDER
CONTACT US TO ORDER
Our promise to you:
Guaranteed product quality, expert customer support.
 24x7 CUSTOMER SERVICE
24x7 CUSTOMER SERVICE
 CONTACT US TO ORDER
CONTACT US TO ORDER
Transporters facilitate the movement of a specific substrate — either with or against its concentration gradient. It is usually believed that conformational change of the transporter protein is important in this transfer process. Transporters move molecules at only about 102 to 104 s21, a rate considerably slower than that associated with channel proteins. Many of these transporters belong to the solute carrier (SLC) gene superfamily-and include passive transporters, symporters and antiporters, as well as mitochondrial and vesicular transporters. SLC transporters can transport solutes, such as ions, metabolites, peptides, and drugs, across biological membranes, using diverse energy coupling mechanisms.
The overview of human SLC superfamily
The human SLC superfamily comprises 386 member proteins that transport a broad spectrum of substrates, such as nutrients, toxins, and prescription drugs. The superfamily members are classified into 52 families, based on the number of predicted or observed transmembrane a-helices (usually 10–14) and sequence similarity, in which members of each family share sequence identity of at least 20% with at least one other family member. Members of an SLC family can have substrates with different physicochemical properties despite their common evolutionary origin. For example, the SLC22 family includes transporters of organic anions, cations, or zwitterions. Conversely, SLC families such as the amino acid transporter families SLC1 and SLC7 can be unrelated evolutionarily but still have chemically similar substrates.
SLC transporters in human physiology
For example, amino acids, which are needed for protein synthesis, are absorbed and reabsorbed by SLC1, SLC3, SLC6, SLC7, SLC25 and SLC36 families’ transporters, many members of which are expressed in the intestine and kidney. In the intestine, amino acids are absorbed from the lumen into the body. And in the kidney, amino acids that are filtered out of the bloodstream by the glomerulus are reabsorbed in the proximal tubule by many of the same transporters that are involved in intestinal absorption. Members of the SLC2, SLC5 and SLC50 families are required for intestinal absorption and renal reabsorption of glucose and for glucose uptake into neurons, erythrocytes, hepatocytes and other cell types. Metals often serve as essential cofactors for important enzymes, but toxicities may occur when they are present at excess concentrations. Zinc transporters of the SLC30 and SLC39 families and iron transporters of the SLC11 and SLC40 families regulate zinc and iron levels in the body, respectively. Similarly, water-soluble vitamins are essential for various processes but require transporters for cellular uptake; for instance, SLC19 family members transport folate and thiamine, SLC46 family members transport folate and the SLC52 family transports riboflavin. In the brain, neurotransmitters released into the synapse are taken back into presynaptic neurons through SLC1 and SLC6 transporters. Interestingly, the roles of many transporters in human physiology have been discovered through studies of Mendelian disease (Figure 1).
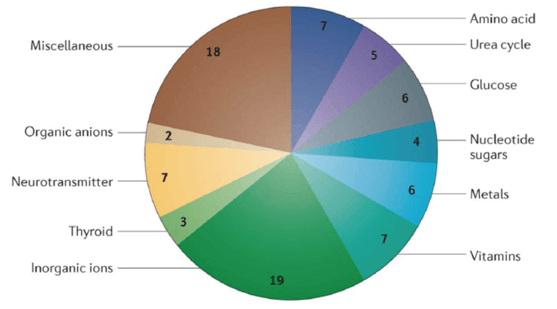
Figure 1. SLC transporters implicated in Mendelian diseases.
SLC transporters in human disease
Given the key physiological roles of SLC transporters, it is not surprising that defects in a single transporter can result in a serious disease. Mutations in 20% of the genes encoding known SLC transporters in humans have been associated with Mendelian disease, and it is likely that mutations in more SLC transporter genes will be found to be causal for some of the remaining half of Mendelian diseases with no known cause. The defective transporters or transporter deficiencies that cause these diseases result in diverse symptoms that affect almost all organ systems; although some of these diseases are considered to be benign, others cause serious illness and death. Figure 2 illustrates the potential mechanisms through which mutations in genes encoding SLC transporters can result in reduced function.
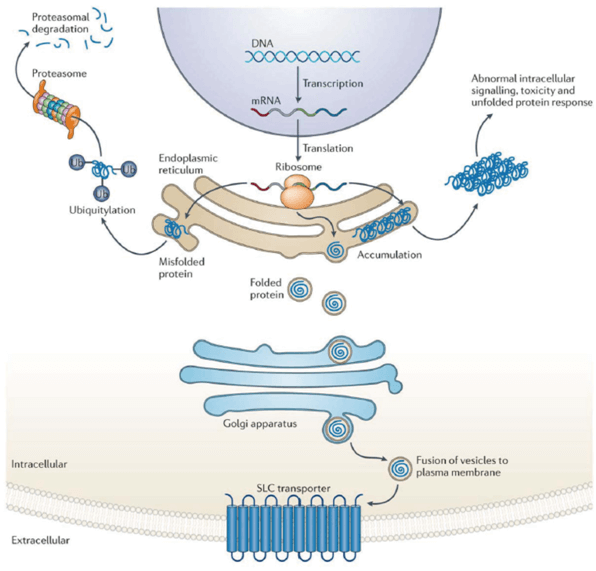
Figure 2. The different types of mutations in SLC transporter genes.
SLC transporter gene polymorphisms that are associated with common disease have usually been identified through the genotyping of candidate genes, or from GWASs. Examples of transporter genes for which polymorphisms have been associated with human traits or disease include the following: SLC22A4 and SLC22A5, which are associated with inflammatory bowel disease; SLC2A9, SLC22A11 and SLC22A12, which are associated with uric acid and gout levels; SLC24A5 and SLC45A2, which are associated with skin color; and SLCO1B1 and SLCO1B3, which are associated with high bilirubin levels. A number of the polymorphisms implicated in human disease are non-synonymous — that is, they cause amino acid substitutions in the encoded transporter that reduce transporter function or expression levels. For example, non-synonymous variants of the uric acid transporters encoded by SLC2A9, SLC22A11 and SLC22A12 show reduced uric acid transport in vitro.
SLC transporters as targets of drugs
Most current drugs — as well as novel drugs in clinical trials - that modulate SLC transporters do so by inhibiting transporter activity. For diseases in which decreased transporter activity leads to a potentially beneficial effect, high-throughput screening (HTS) of large compound libraries by using cell lines that overexpress the transporter of interest can be used to discover lead inhibitor molecules. SLC transporters represent a plethora of new therapeutic targets for rare diseases, and may be particularly amenable to targeting with small molecules. Importantly, many SLC transporters are expressed on the cell surface and are therefore targetable by both small molecules and therapeutic antibodies. There are four approved drug classes for which the primary mechanisms of action involve inhibition of SLC transporters: diuretics, glucose transporter inhibitors for diabetes, neurotransmitter-reuptake inhibitors for neuropsychiatric indications and uric acid transporter inhibitors for gout.
Drug ADME and SLC families
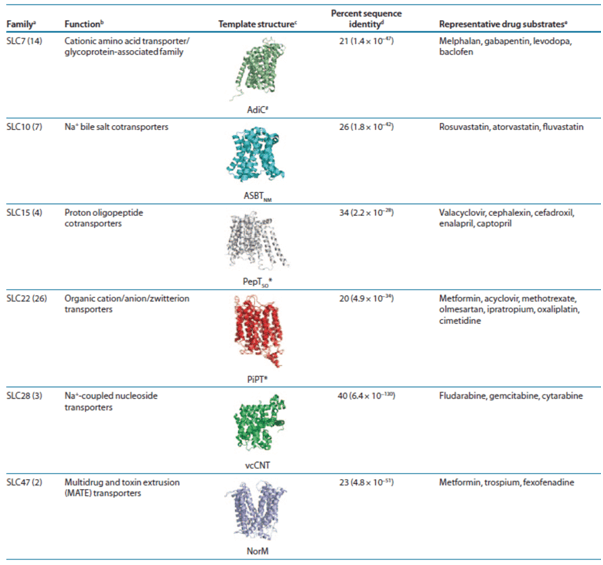
Atomic structures of SLC transporters can guide computations and experiments to describe the mechanism of transport and its clinical implications. In particular, structures can be used to rationalize the effect of nonsynonymous variants on drug transport, pharmacokinetics, or pharmacodynamics, as well as to predict these functional consequences of uncharacterized variants. Moreover, the transporter structure can be used to predict small-molecule ligands such as endogenous metabolites, fragment molecules, and prescription drugs via virtual screening of compound libraries, which can contain millions of purchasable molecules, against the transporter binding site. The ligand prediction is usually followed by experimental testing, and the confirmed hits can then (i) guide drug–drug interaction studies, (ii) provide novel chemical tools to further characterize the transporters’ functions, and even (iii) serve as leads for designing drugs targeting selected SLC transporters. Several structures of SLC transporters from various prokaryotic and eukaryotic organisms can serve as templates for modeling the human members (Table 1).
Table 1. Drug ADME SLC families that can be modeled based on atomic resolution structures.
Conclusions
Although 92% of known drug-target structures have been deposited in the Protein Data Bank, most SLC transporters have not been crystallized, thus limiting computer-aided drug discovery efforts. Moreover, many SLC transporters remain orphans, with unknown function and unknown substrates. Finally, SLC transporters located in intracellular compartments may not be as amenable to drug targeting. For example, to our knowledge, the SLC25 family of mitochondrial transporters, some of which are associated with Mendelian disease, have not yet been targeted. Despite these obstacles, the potential for discovering inhibitors of transporters or modulators of transporter activity is enormous. The development of small molecules that can target specific variants of the ABC transporter CFTR suggests that efforts to target mutant SLC transporters may be possible. A comprehensive understanding of the mechanisms responsible for rare and common diseases — including the function of the transporters and the pathways in which they act -will be crucial for recognizing other proteins that may be therapeutically targeted.
References: