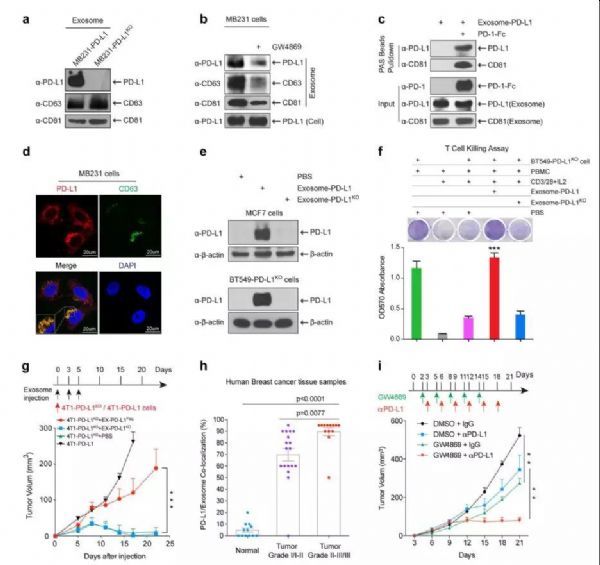
PD-L1 is a classical immunological surface protein that inhibits the anti-tumor function of T cells by binding to receptor programmed cell death-1 (PD-1) and effectively protects tumors from immune surveillance. For estimating the role of exo-PD-L1 in the tumor microenvironment and tumor progression in vivo, researchers measured tumor growth in 4T1-PD-L1 KO cells co-injected with EX-PD-L1 Flag, EX-PD-L1 KO or PBS. Consistent with previous reports, PD-L1 deficiency in 4T1-PD-L1 KO cells resulted in significant tumor regression, however, EX-PD-L1 Flag, but not EX-PD-L1 KO, significantly restored tumor growth in 4T1-PD-L1 KO cells. Then they exposed 4T1-PD-L1 KO cells to increasing amounts of EX-PD-L1 Flag, and the results showed that EX-PD-L1 Flag promoted tumor growth in a dose-dependent manner.
In addition, 4T1-PD-L1 KO tumors co-injected with EX-PD-L1 Flag showed less granzyme B expression than co-injection with EX-PD-L1 KO or PBS, indicating a decrease in cytotoxic T cell activity in the tumor area. These results indicate that EX-PD-L1 protects and promotes tumor growth in vivo by inhibiting the secretion of T cell granzyme B. These findings are consistent with the above-mentioned in vitro studies, in vivo results also demonstrated that exosome PD-L1 binds to PD-1 and inhibits the killing activity of T cell. The results of IHC staining showed colocalization of PD-L1 and the exosome marker CD63 in MVB in human tumor tissue samples, researchers sought to further determine whether their co-localization was related to the development stage of the patient's breast cancer. Analysis of a panel of human breast cancer tissue microarrays revealed a higher level of PD-L1-CD63 colocalization associated with higher stages of disease, which is consistent with recent reports of PD-L1 levels in exosomes. Based on the above results, they hypothesized that the growing tumor continues to secrete exo-PD-L1 into the tumor microenvironment to inactivate T cell function, which may induce immunotherapy resistance.
Researchers treated tumors in a mouse model with a combination of the exosomal secretion inhibitor GW4869 and PD-L1 antibodies to explore the possibility of targeting exosomes to enhance the efficacy of immunotherapy. First, the results show that GW4869 inhibits exocrine secretion of 4T1 mouse breast cancer cells. Inhibition of GW4869 exocytosis inhibited 4T1 tumor growth in BALB/c mice, but 4T1 cell growth was not inhibited in vitro and in immunodeficient nude mice, suggesting that treatment with GW4869 will not inhibit 4T1 tumor growth, but may inhibit anti-tumor immunity, including inhibition of exo-PD-L1 secretion. As expected, GW4869 treatment significantly enhanced the anti-PD-L1 therapeutic efficacy in 4T1 tumor growth inhibition. Furthermore, they constructed a Tet-on-inducible Rab27a knockdown 4T1 cell line in order to specifically inhibit exocrine secretion of 4T1 cells. Consistent with previous reports, knockdown of Rab27a via Dox treatment significantly inhibited exocrine secretion, and the results further showed that knockdown of Rab27a significantly inhibited 4T1 tumor growth. Similar to GW4869 treatment, specific inhibition of exosome secretion by Rab27a knockdown in tumor cells significantly enhanced the efficacy of anti-PD-L1 treatment. Thus, inhibition of exosome secretion by pharmacological inhibitors (GW4869) and genetic methods (Rab27a knockdown) in tumor cells significantly inhibited 4T1 tumor growth and enhanced anti-PD-L1 therapeutic efficacy. It is worth mentioning that the tumor inhibition is relatively far-reaching compared with anti-PD-L1 antibody treatment by knocking out Rab27a or inhibitor GW4869, indicating that blockade of exocytic PD-L1 secreted in tumor cells significantly promotes anti-tumor immunity. In addition, the combination of the two leads to stronger tumor suppression.
Although exosomes have been proved to positively transport PD-L1 from PD-L1 to PD-L1-negative breast cancer cells, and exo-PD-L1 binds to PD-1 on T cells to inhibit T cell activation and killing activity, human bone marrow APC has also been reported to express PD-L1 and has an important contribution to immunotherapy. Therefore, the researchers also tested whether exosomes containing PD-L1 can transport PD-L1 to bone marrow APC. They detected the transfer of PD-L1 from exo-PD-L1 to the human monocyte cell line THP1 (a precursor of macrophages and DCs). The results of flow cytometry analysis further revealed that exosomes can transfer PD-L1 EGFP to DCs in human macrophages and PBMCs in vitro. In vivo, IF staining showed that the EX-PD-L1 Flag positively stained the tumor-infiltrating macrophages PD-L1 Flag, however, they did not observe positive staining in DCs. It is unclear why the efficiency of transfer of PD-L1 to DC by exosomes in vivo is lower than that of macrophages, which may be due to a lower amount of DC and/or some unknown mechanism that reduces efficiency in the tumor microenvironment. However, the results indicate that PD-L1 can be transferred to a variety of cell types, including tumor cells, macrophages, and DCs (at least in vitro) by exosomes containing PD-L1 in the tumor microenvironment. Thus, exosomes appear to act as transport carriers to deliver PD-L1 to different cell types in order to modulate immune surveillance in the tumor microenvironment. Although it is not clear whether macrophages or DCs expressing PD-L1 may also secrete exosomes-PD-L1, however, the results showed that blocking of exocytic secretion in Rab27a knockout tumor cells inhibited the growth of 4T1 tumors, and GW4869 treatment had a similar effect. This study shows that exosomes PD-L1 from tumor cells play an important role in the regulation of immune regulation of the tumor microenvironment.
Overall, current findings suggest that PD-L1 exerts a passive protective effect through its presence in exosomes and also plays a role in active defense, and exosomes containing PD-L1 attenuate anti-tumor immunity in the tumor microenvironment. Furthermore, the results indicate that blockade of exocytosis of PD-L1 secretion contributes to anti-tumor immunity and that the combination of exocytosis inhibition and anti-PD-L1 treatment has the potential to improve the anti-tumor response in the clinic.