For a very long time, it has been thought that the neurodegenerative disorder of Alzheimer's disease is caused by β-amyloid plaques, which is called Amyloid Precursor Protein (APP), breaking down into fragments and accumulates in the brain to form misfolded, toxic polymers that impede neural communication. In the presence of Alzheimer's disease, β-amyloid plaques will lead to neuronal death directly, or destroy the nutritional supply of brain cells through the action of tau protein phosphorylation (tau protein bending to form neurofibrillary tangles), eventually killing them. However, a new study recently published in Stem Cell Reports by the University of Queensland, Australia suggested that we may need to rethink the biological mechanisms that lead to cognitive decline in Alzheimer's disease.
Ernst Wolvetang, the stem cell biologist at the Institute of Bio-engineering and Nanotechnology, said that, ‘Our data challenge the current research findings in this field, that is, β-amyloid plaques are sufficient to cause neurodegenerative changes associated with Alzheimer's disease.’
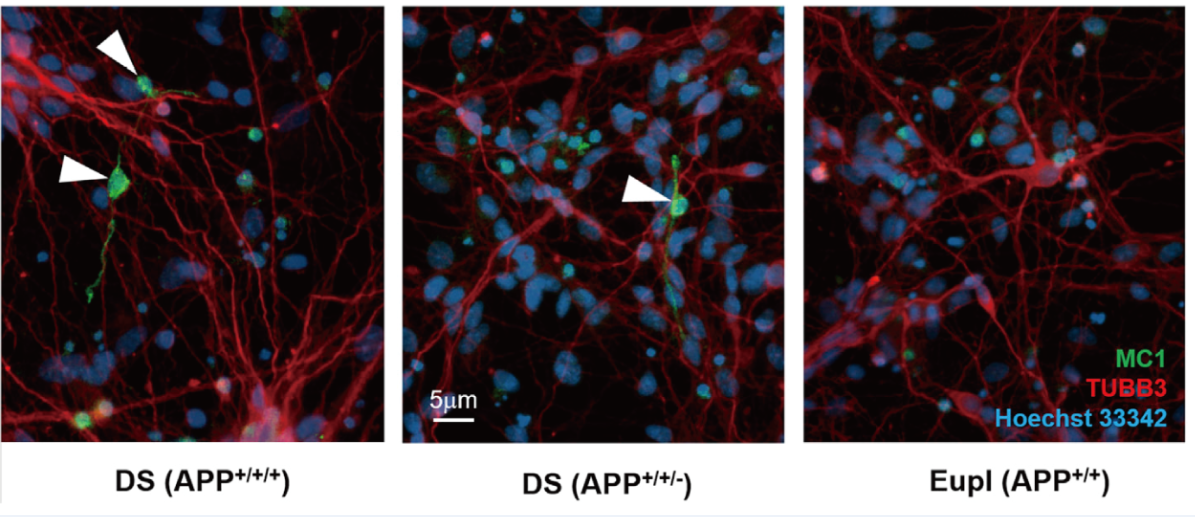
Detection of pathological tau with the conformation-specific antibody MC1 revealed potential early NFT-like structures in day 120 DS neurons that were absent in isogenic euploid neurons but persisted in DS APP+/+/- neuronal cultures (Ovchinnikov et al., Stem Cell Reports, 2018)
The Wolvetang team utilized stem cells from patients with Down Syndrome in humans to study these mechanisms. These patients had one more copy of the amyloid precursor protein (APP) gene because they had an extra chromosome 21. The increase in APP levels is considered to be responsible for Alzheimer's disease in patients with Down syndrome. But when researchers cultured stem cells into neurons in vitro, and then applying CRISPR gene editing technology to manipulate APP back to normal levels, there isn’t any change observed in tau phosphorylation.
Although these experiments proved that increased APP levels will result in increased β-amyloid plaques, however, it can not cause an increase in the number of neuronal deaths and tau protein toxic neurofibrillary tangles by itself.
Wolvetang explained, ‘This suggests that β-amyloid may not be a driving factor of neuronal cell death associated with Alzheimer's disease, nor would it be the pathological state that leads to tau protein directly, at least in our model. What I want to emphasize is that we have not over-interpreted these data. Despite this, we have spent decades and billions of dollars studying therapies based on β-amyloid, and almost all failed so far. Many studies have shown that this traditional assumption may need to be reassessed, and our data further supports this insight.’
However, if the levels of β-amyloid and APP are not directly related to the entanglement of tau proteins and cognitive decline, then what causes neuronal cell death in patients with Alzheimer's disease? The researchers cannot confirm the reason at present, but they believe that new research should pay more attention to the process and pathology of tau protein because β-amyloid may not be involved in the disease as what has been believed in the past.
Wolvetang explained, 'In the context of Down Syndrome, we focused on other genes encoded by chromosome 21, such as DYRK1A, which directly or indirectly leads to an increase in tau phosphorylation, is also associated with Alzheimer's disease.'
Although researchers have carefully emphasized the limitations of their research, such as those human cells based on cultivation, may not be able to prove that this is effective for real Alzheimer patients, they still expect that this method will help develop new therapies in the near future.
Wolvetang said, 'This study emphasizes that the disease model based on human stem cells can provide us with new insights into the molecular mechanisms that lead to Alzheimer's disease, and open up a new path for drug screening.'
Reference:
Ovchinnikov et al., The Impact of APP on Alzheimer-like Pathogenesis and Gene Expression in Down Syndrome iPSC-Derived Neurons. Stem Cell Reports, 2018.